|
Brownian Motion Of Sol Particles
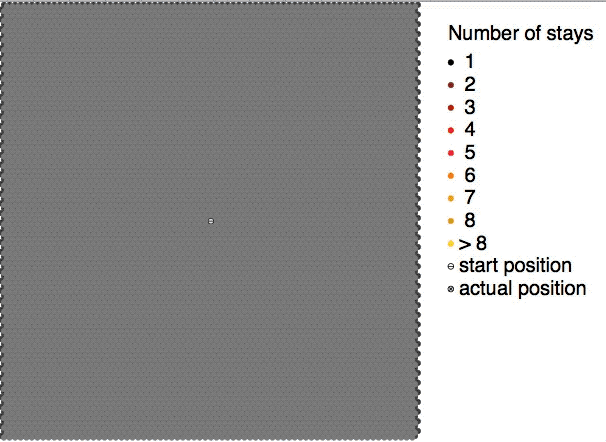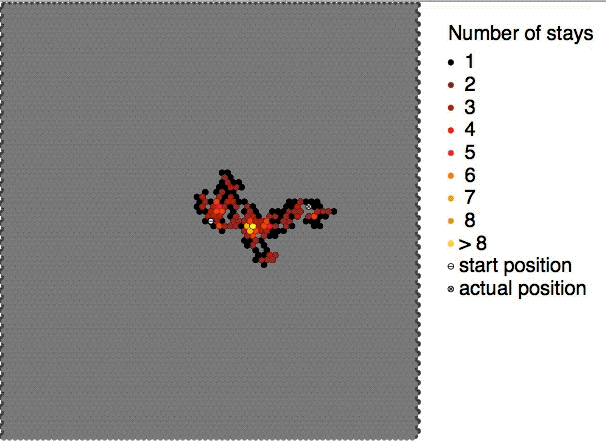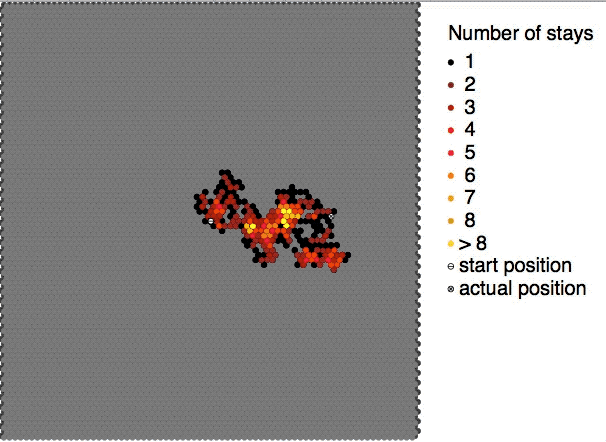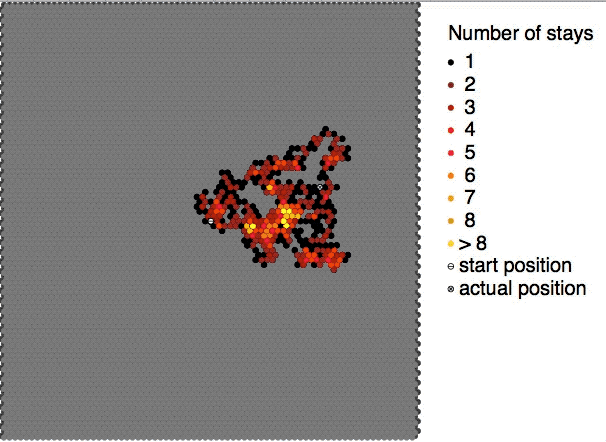
Colloidal particles in a sol are continuously bombarded by the molecules of the dispersion medium on all sides. The impacts are however not equal in every direction. As a result, the sol particles show random or zig-zag movements. This random or zig-zag motion of the colloidal particles in a sol is called Brownian motion or Brownian movement. The phenomenon of Brownian motion was observed by Robert Brown in the form of random zig-zag motion of pollen grains suspended in water. This kind of movement is found in all colloidal systems. Such random motion is visible under ultramicroscopes and for bigger particles even under ordinary microscopes. The Brownian motion becomes progressively less prominent, as the particles grow in size or the viscosity The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word ''suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Itali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sol (colloid)
A sol is a colloidal suspension made out of tiny solid particles in a continuous liquid medium. Sols are stable and exhibit the Tyndall effect, which is the scattering of light by the particles in the colloid. Examples include amongst others blood, pigmented ink, cell fluids, paint, antacids and mud. Artificial sols can be prepared by two main methods: dispersion and condensation. In the dispersion method, solid particles are reduced to colloidal dimensions through techniques such as ball milling and Bredig's arc method. In the condensation method, small particles are formed from larger molecules through a chemical reaction. The stability of sols can be maintained through the use of dispersing agents, which prevent the particles from clumping together or settling out of the suspension. Sols are often used in the sol-gel process, in which a sol is converted into a gel through the addition of a crosslinking agent. In a sol, the dispersed phase is typically solid and the continu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dispersion Medium
Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with colloids, heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a true solution and a suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane. Interface and colloid science has applications and ramifications in the chemical industry, pharmaceuticals, biotechnology, ceramics, minerals, nanotechnology, and microfluidics, among others. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brownian Motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas). This pattern of motion typically consists of random fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium, defined by a given temperature. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena). More specifically, the fluid's overall linear and angular momenta remain null over time. The kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's internal energy (the equipartition theorem). This motion is named after the botanist Robert Brown, who first described the phenomenon in 1827, while looking throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Brown (botanist, Born 1773)
Robert Brown (21 December 1773 – 10 June 1858) was a Scottish botanist and paleobotanist who made important contributions to botany largely through his pioneering use of the microscope. His contributions include one of the earliest detailed descriptions of the cell nucleus and cytoplasmic streaming; the observation of Brownian motion; early work on plant pollination and fertilisation, including being the first to recognise the fundamental difference between gymnosperms and angiosperms; and some of the earliest studies in palynology. He also made numerous contributions to plant taxonomy, notably erecting a number of plant families that are still accepted today; and numerous Australian plant genera and species, the fruit of his exploration of that continent with Matthew Flinders. Early life Robert Brown was born in Montrose on 21 December 1773, in a house that existed on the site where Montrose Library currently stands. He was the son of James Brown, a minister in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultramicroscope
An ultramicroscope is a microscope with a system that lights the object in a way that allows viewing of tiny particles via light scattering, and not light reflection or absorption. When the diameter of a particle is below or near the wavelength of visible light (around 500 nanometers), the particle cannot be seen in a light microscope with the usual methods of illumination. The ''ultra-'' in ''ultramicroscope'' refers to the ability to see objects whose diameter is shorter than the wavelength of visible light, on the model of the ''ultra-'' in ''ultraviolet''. Synopsis In the system, the particles to be observed are dispersed in a liquid or gas colloid (or less often in a coarser suspension). The colloid is placed in a light-absorbing, dark enclosure, and illuminated with a convergent beam of intense light entering from one side. Light hitting the colloid particles will be scattered. In discussions about light scattering, the converging beam is called a "Tyndall cone". The scene is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloidal Chemistry
Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with colloids, heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a true solution and a suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane. Interface and colloid science has applications and ramifications in the chemical industry, pharmaceuticals, biotechnology, ceramics, minerals, nanotechnology, and microfluidics, among others. There ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
