|
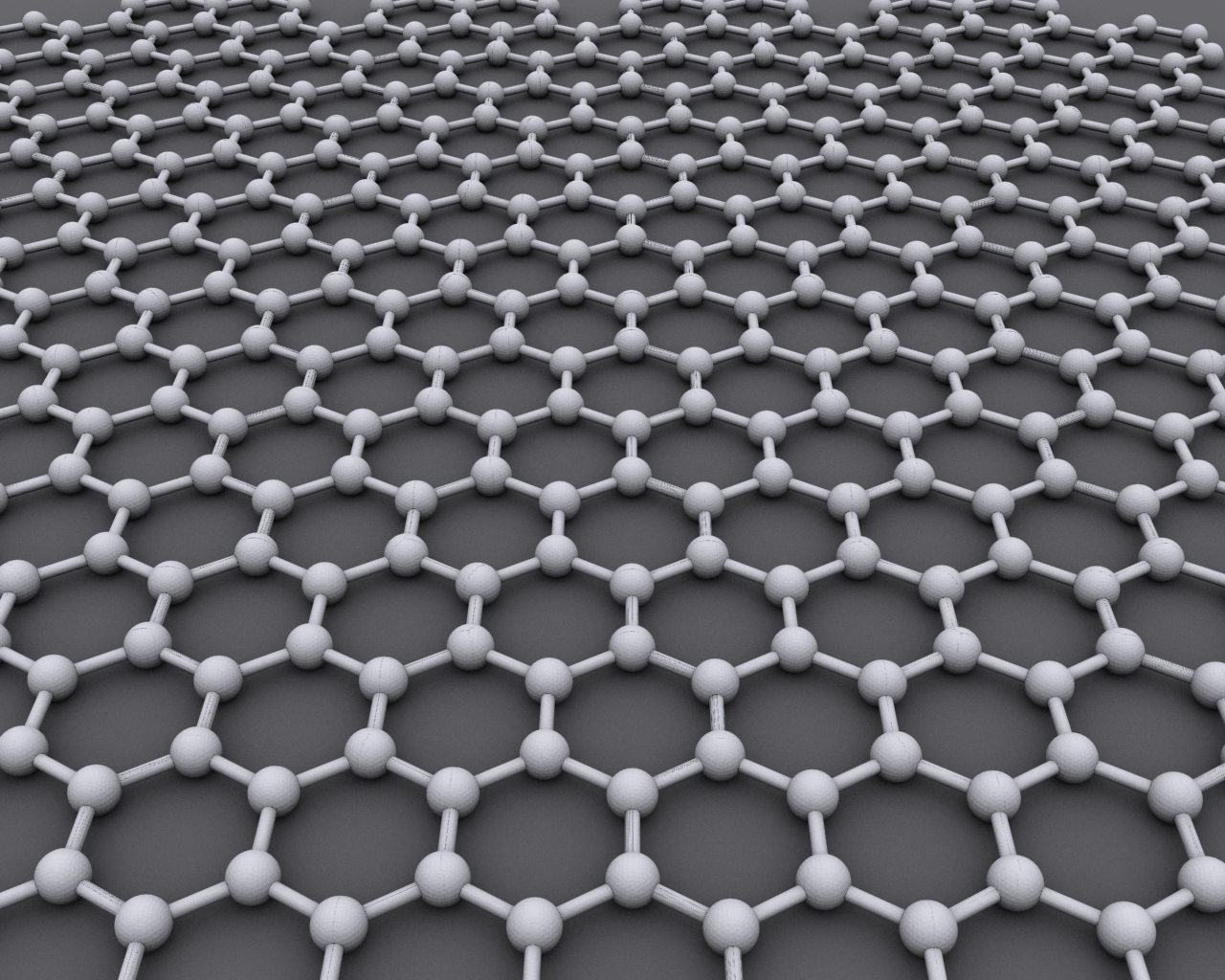
Boron Nitride Nanosheet
Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal boron nitride (h-BN), which has a thickness of one to few atomic layers. It is similar in geometry as well as physical and thermal properties to its all-carbon analog graphene, but has very different chemical and electronic properties – contrary to the black and highly conducting graphene, BN nanosheets are Insulator (electricity), electrical insulators with a band gap of ~5.9 eV, and therefore appear white in color. Uniform monoatomic BN nanosheets can be deposited by catalytic decomposition of borazine at a temperature ~1100 °C in a chemical vapor deposition setup, over substrate areas up to about 10 cm2. Owing to their hexagonal atomic structure, small lattice mismatch with graphene (~2%), and high uniformity they are used as substrates for graphene-based devices. Structure BN nanosheets consist of sp2-conjugated boron and nitrogen atoms that form a honeycomb structure. They contain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride Nanotube
Boron nitride nanotubes (BNNTs) are a polymorph of boron nitride. They were predicted in 1994 and experimentally discovered in 1995. Structurally they are similar to carbon nanotubes, which are cylinders with sub-micrometer diameters and micrometer lengths, except that carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology. In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure. BNNTs have unique physical and chemical properties, when compared to Carbon Nanotubes (CNTs) providing a very wide range of commercial and scientific applications. Although BNNTs and CNTs share similar tensile strength properties of circa 100 times s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Two-dimensional Nanomaterials
In materials science, the term single-layer materials or 2D materials refers to crystalline solids consisting of a single layer of atoms. These materials are promising for some applications but remain the focus of research. Single-layer materials derived from single elements generally carry the -ene suffix in their names, e.g. graphene. Single-layer materials that are compounds of two or more elements have -ane or -ide suffixes. 2D materials can generally be categorized as either 2D allotropes of various elements or as compounds (consisting of two or more covalently bonding elements). It is predicted that there are hundreds of stable single-layer materials. The atomic structure and calculated basic properties of these and many other potentially synthesisable single-layer materials, can be found in computational databases. 2D materials can be produced using mainly two approaches: top-down exfoliation and bottom-up synthesis. The exfoliation methods include sonication, mechanical, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Electrolysis
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, which is used in welding and other applications. Electrolysis of water requires a minimum potential difference of 1.23 volts, though at that voltage external heat is required. E lectrolysis is rarely used in industrial applications since hydrogen can be produced less expensively from fossil fuels. History In 1789, Jan Rudolph Deiman and Adriaan Paets van Troostwijk used an electrostatic machine to make electricity that was discharged on gold electrodes in a Leyden jar with water. In 1800 Alessandro Volta invented the voltaic pile, and a few weeks later English scientists William Nicholson and Anthony Carlisle used it to electrolyse water. In 1806 Humphry Davy reported the results of ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most battery (electricity), batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Robert Grove, William Grove in 1838. The first commercial use of fuel cells came more than a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for sat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
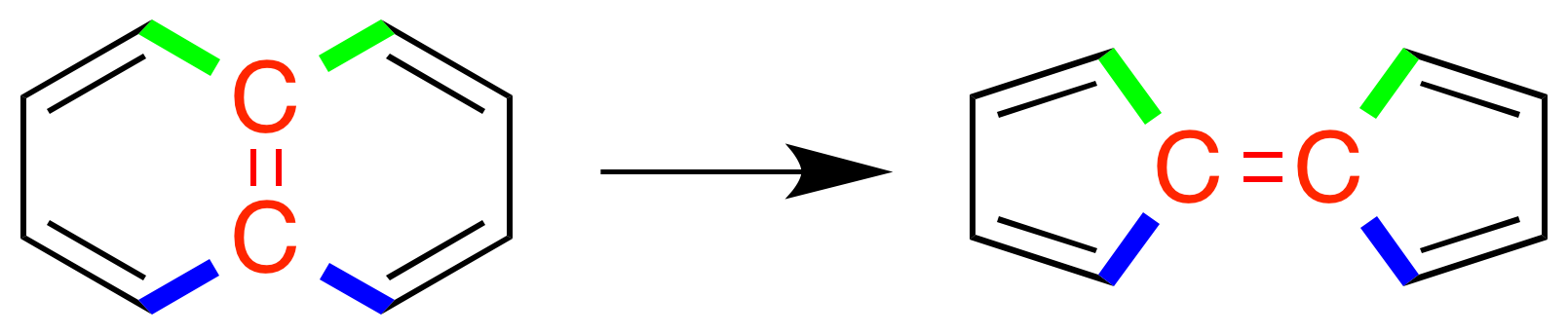
Stone–Wales Defect
A Stone–Wales defect is a crystallographic defect that involves the change of connectivity of two pi bond, π-bonded carbon atoms, leading to their rotation by 90° with respect to the midpoint of their bond. The reaction commonly involves conversion between a naphthalene-like structure into a fulvalene-like structure, that is, two rings that share an edge vs two separate rings that have vertices bonded to each other. The reaction occurs on carbon nanotubes, graphene, and similar carbon frameworks, where the four adjacent six-membered rings of a pyrene-like region are changed into two five-membered rings and two seven-membered rings when the bond uniting two of the adjacent rings rotates. In these materials, the rearrangement is thought to have important implications for the thermal, chemical, electrical, and mechanical properties. The rearrangement is an example of a pyracyclene rearrangement. History The defect is named after Anthony Stone and David J. Wales at the Univers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important raw material for the chemical industry. In 1828 Friedrich Wöhler discovered that urea can be produced from inorganic starting materials, which was an important conceptual milestone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxoacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However boric acid and borates have been used since the time of the ancient Greeks for cleaning, preserving food, and other activities. Molecular a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energetically favorable than the bulk of the material (the atoms on the surface have more energy compared with the atoms in the bulk), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. There is "excess energy" as a result of the now-incomplete, unrealized bonding at the two surfaces. Cutting a solid body into pieces disrupts its bonds and increases the surface area, and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-Dimethylfuran
2,5-Dimethylfuran is a heterocyclic compound with the formula (CH3)2C4H2O. Although often abbreviated DMF, it should not be confused with dimethylformamide. A derivative of furan, this simple compound is a potential biofuel, being derivable from cellulose. Production Fructose can be converted into 2,5-dimethylfuran in a catalytic biomass-to-liquid process. The conversion of fructose to DMF proceeds via hydroxymethylfurfural. Fructose is obtainable from glucose, a building block in cellulose. Potential as a biofuel DMF has a number of attractions as a biofuel. It has an energy density 40% greater than that of ethanol, making it comparable to gasoline (petrol). It is also chemically stable and, being insoluble in water, does not absorb moisture from the atmosphere. Evaporating dimethylfuran during the production process also requires around one third less energy than the evaporation of ethanol, although it has a boiling point some 14 °C higher, at 92 °C, compared to 78& ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-side-3D-balls.png)