Boron nitride nanosheet on:
[Wikipedia]
[Google]
[Amazon]
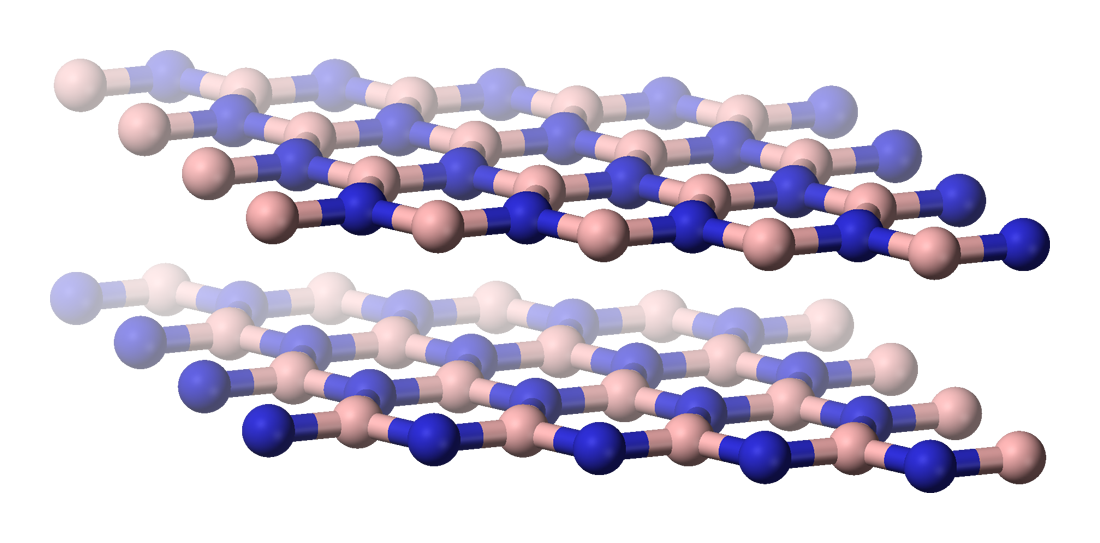
 Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal
Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal
 Mechanical cleaving methods of boron nitride use shear forces to break the weak van der Waals interactions between the BN layers. Cleaved nanosheets have low defect densities and retain the lateral size of the original substrate. Inspired by its use in the isolation of graphene, micromechanical cleavage, also known as the Scotch-tape method, has been used to consistently isolate few-layer and monolayer boron nitride nanosheets by subsequent exfoliation of the starting material with adhesive tape. The disadvantage of this technique is that it is not scalable for large-scale production.
Boron nitride sheets can be also exfoliated by
Mechanical cleaving methods of boron nitride use shear forces to break the weak van der Waals interactions between the BN layers. Cleaved nanosheets have low defect densities and retain the lateral size of the original substrate. Inspired by its use in the isolation of graphene, micromechanical cleavage, also known as the Scotch-tape method, has been used to consistently isolate few-layer and monolayer boron nitride nanosheets by subsequent exfoliation of the starting material with adhesive tape. The disadvantage of this technique is that it is not scalable for large-scale production.
Boron nitride sheets can be also exfoliated by

 Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal
Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal boron nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal ...
(h-BN), which has a thickness of one to few atomic layers. It is similar in geometry as well as physical and thermal properties to its all-carbon analog graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
, but has very different chemical and electronic properties – contrary to the black and highly conducting graphene, BN nanosheets are Insulator (electricity), electrical insulators with a band gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in ...
of ~5.9 eV, and therefore appear white in color.
Uniform monoatomic BN nanosheets can be deposited by catalytic decomposition of borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this ...
at a temperature ~1100 °C in a chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (substra ...
setup, over substrate areas up to about 10 cm2. Owing to their hexagonal atomic structure, small lattice mismatch with graphene (~2%), and high uniformity they are used as substrates for graphene-based devices.
Structure
BN nanosheets consist of sp2-conjugatedboron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atoms that form a honeycomb structure. They contain two different edges: armchair and zig-zag. The armchair edge consists of either boron or nitrogen atoms, while the zig-zag edge consists of alternating boron and nitrogen atoms. These 2D structures can stack on top of each other and are held by Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s to form few-layer boron nitride nanosheets. In these structures, the boron atoms of one sheet are positioned on top or below the nitrogen atoms due to electron-deficient nature of boron and electron-rich nature of nitrogen.
Synthesis
CVD
Chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (substra ...
is the most common method to produce BN nanosheets because it is a well-established and highly controllable process that yields high-quality material over areas exceeding 10 cm2. There is a wide range of boron and nitride precursors for CVD synthesis, such as borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this ...
, and their selection depends on toxicity, stability, reactivity, and the nature of the CVD method.
Mechanical cleavage
 Mechanical cleaving methods of boron nitride use shear forces to break the weak van der Waals interactions between the BN layers. Cleaved nanosheets have low defect densities and retain the lateral size of the original substrate. Inspired by its use in the isolation of graphene, micromechanical cleavage, also known as the Scotch-tape method, has been used to consistently isolate few-layer and monolayer boron nitride nanosheets by subsequent exfoliation of the starting material with adhesive tape. The disadvantage of this technique is that it is not scalable for large-scale production.
Boron nitride sheets can be also exfoliated by
Mechanical cleaving methods of boron nitride use shear forces to break the weak van der Waals interactions between the BN layers. Cleaved nanosheets have low defect densities and retain the lateral size of the original substrate. Inspired by its use in the isolation of graphene, micromechanical cleavage, also known as the Scotch-tape method, has been used to consistently isolate few-layer and monolayer boron nitride nanosheets by subsequent exfoliation of the starting material with adhesive tape. The disadvantage of this technique is that it is not scalable for large-scale production.
Boron nitride sheets can be also exfoliated by ball milling
A ball mill is a type of grinder used to grind or blend materials for use in mineral dressing processes, paints, pyrotechnics, ceramics, and selective laser sintering. It works on the principle of impact and attrition: size reduction is done ...
, where shear forces are applied on the face of bulk boron nitride by rolling balls. This technique yields large quantities of low-quality material with poor control over its properties.
Unzipping of boron nitride nanotubes
BN nanosheets can be synthesized by the unzippingboron nitride nanotube
Boron nitride nanotubes (BNNTs) are a polymorph of boron nitride. They were predicted in 1994 and experimentally discovered in 1995. Structurally they are similar to carbon nanotubes, which are cylinders with sub-micrometer diameters and micromete ...
s via potassium intercalation or etching by plasma or an inert gas. Here the intercalation method has a relatively low yield as boron nitride is resistive to the effects of intercalants. In situ unzipping of boron nitride nanotubes to nanoribbons was achieved by Li et al.
Solvent exfoliation and sonication
Solvent exfoliation is often used in tandem withsonication
A sonicator at the Weizmann Institute of Science during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seawe ...
to isolate large quantities of boron nitride nanosheets. Polar solvents such as isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
and DMF are more effective in exfoliating boron nitride layers than nonpolar solvents because these solvents possess a similar surface energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
to the surface energy of boron nitride nanosheets. Combinations of different solvents also exfoliate boron nitride better than individual solvents. Many solvents suitable for BN exfoliation are rather toxic and expensive, but they can be replaced by water and isopropyl alcohol without significantly sacrificing the yield.
Chemical functionalization and sonication
Chemical functionalization of boron nitride involves attaching molecules onto the outer and inner layers of bulk boron nitride. There are three types of BN functionalization: covalent, ionic and or non-covalent. Layers are exfoliated by placing the functionalized BN into a solvent and allowing the solvation force between the attached groups and the solvent to break the van der Waal forces between BN layers. This method is slightly different from solvent exfoliation, which relies on the similarities between the surface energies of the solvent and boron nitride layers.Solid state reactions
Heating a mixture of boron and nitrogen precursors, such asboric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves ...
and urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, can produce boron nitride nanosheets. The number of layers in these nanosheets was controlled by temperature (ca. 900 ˚C) and the urea content.
Properties and applications
Mechanical properties. Monolayer boron nitride has an average Young's modulus of 0.865 TPa and fracture strength of 70.5 GPa. In contrast to graphene, whose strength decreases dramatically with increased thickness, few-layer boron nitride sheets have a strength similar to that of monolayer boron nitride. Thermal conductivity. The thermal conductivity of atomically thin boron nitride is one of the highest among semiconductors and electrical insulators; it increases with reduced thickness due to less intra-layer coupling. Thermal stability. The air stability of graphene shows a clear thickness dependence: monolayer graphene is reactive to oxygen at 250 °C, strongly doped at 300 °C, and etched at 450 °C; in contrast, bulk graphite is not oxidized until 800 °C. Atomically thin boron nitride has much better oxidation resistance than graphene. Monolayer boron nitride is not oxidized till 700 °C and can sustain up to 850 °C in air; bilayer and trilayer boron nitride nanosheets have slightly higher oxidation starting temperatures. The excellent thermal stability, high impermeability to gas and liquid, and electrical insulation make atomically thin boron nitride potential coating materials for preventing surface oxidation and corrosion of metals and other two-dimensional (2D) materials, such as black phosphorus. Better surface adsorption. Atomically thin boron nitride has been found to have better surface adsorption capabilities than bulk hexagonal boron nitride. According to theoretical and experimental studies, atomically thin boron nitride as an adsorbent experiences conformational changes upon surface adsorption of molecules, increasing adsorption energy and efficiency. The synergic effect of the atomic thickness, high flexibility, stronger surface adsorption capability, electrical insulation, impermeability, high thermal and chemical stability of BN nanosheets can increase the Raman sensitivity by up to two orders, and in the meantime attain long-term stability and extraordinary reusability not achievable by other materials. Dielectric properties. Atomically thin hexagonal boron nitride is an excellent dielectric substrate for graphene, molybdenum disulphide (MoS2), and many other 2D material-based electronic and photonic devices. As shown by electric force microscopy (EFM) studies, the electric field screening in atomically thin boron nitride shows a weak dependence on thickness, which is in line with the smooth decay of electric field inside few-layer boron nitride revealed by the first-principles calculations. Raman characteristics. Raman spectroscopy has been a useful tool to study a variety of 2D materials, and the Raman signature of high-quality atomically thin boron nitride was first reported by Gorbachev et al. and Li et al. However, the two reported Raman results of monolayer boron nitride did not agree with each other. Cai et al. conducted systematic experimental and theoretical studies of the intrinsic Raman spectrum of atomically thin boron nitride. They reveal that, in absence of interaction with a substrate, atomically thin boron nitride has a G-band frequency similar to that of bulk hexagonal boron nitride, but strain induced by the substrate can cause Raman shifts. Nevertheless, the Raman intensity of G-band can be used to estimate layer thickness and sample quality. BN nanosheets are electrical insulators and have a wide band gap of ~5.9 eV, which can be changed by the presence ofStone–Wales defect
A Stone–Wales defect is a crystallographic defect that involves the change of connectivity of two pi bond, π-bonded carbon atoms, leading to their rotation by 90° with respect to the midpoint of their bond. The reaction commonly involves conve ...
s within the structure, by doping or functionalization, or by changing the number of layers. Owing to their hexagonal atomic structure, small lattice mismatch with graphene (~2%), and high uniformity, BN nanosheets are used as substrates for graphene-based devices. BN nanosheets are also excellent proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
conductors. Their high proton transport rate, combined with the high electrical resistance, may lead to applications in fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
and water electrolysis
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
.
References
{{reflist, refs= {{cite journal, doi=10.1038/ncomms9849, pmid=26611437, pmc=4674780, title=Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization, journal=Nature Communications, volume=6, pages=8849, year=2015, last1=Lei, first1=Weiwei, last2=Mochalin, first2=Vadym N., last3=Liu, first3=Dan, last4=Qin, first4=Si, last5=Gogotsi, first5=Yury, last6=Chen, first6=Ying, bibcode=2015NatCo...6.8849L {{cite journal, doi=10.1021/nn503140y, pmid=25094030, title=Large-Area Monolayer Hexagonal Boron Nitride on Pt Foil, journal=ACS Nano, volume=8, issue=8, pages=8520–8, year=2014, last1=Park, first1=Ji-Hoon, last2=Park, first2=Jin Cheol, last3=Yun, first3=Seok Joon, last4=Kim, first4=Hyun, last5=Luong, first5=Dinh Hoa, last6=Kim, first6=Soo Min, last7=Choi, first7=Soo Ho, last8=Yang, first8=Woochul, last9=Kong, first9=Jing, last10=Kim, first10=Ki Kang, last11=Lee, first11=Young Hee {{cite journal, doi=10.1038/srep16159, pmid=26537788, pmc=4633619, year=2015, last1=Wu, first1=Q, title=Single Crystalline Film of Hexagonal Boron Nitride Atomic Monolayer by Controlling Nucleation Seeds and Domains, journal=Scientific Reports, volume=5, pages=16159, last2=Park, first2=J. H., last3=Park, first3=S, last4=Jung, first4=S. J., last5=Suh, first5=H, last6=Park, first6=N, last7=Wongwiriyapan, first7=W, last8=Lee, first8=S, last9=Lee, first9=Y. H., last10=Song, first10=Y. J., bibcode=2015NatSR...516159W {{cite journal , author = Hu, S. , display-authors=etal , title = Proton transport through one-atom-thick crystals , journal = Nature , year = 2014 , volume = 516 , issue = 7530 , pages = 227–230 , doi = 10.1038/nature14015, pmid=25470058 , arxiv = 1410.8724 , bibcode = 2014Natur.516..227H , s2cid=4455321 {{Cite journal, last1=Lin, first1=Yi, last2=Connell, first2=John W., date=2012, title=Advances in 2D boron nitride nanostructures: nanosheets, nanoribbons, nanomeshes, and hybrids with graphene, journal=Nanoscale, volume=4, issue=22, pages=6908–39, doi=10.1039/c2nr32201c, pmid=23023445, bibcode=2012Nanos...4.6908L {{Cite journal, last1=Li, first1=Lu Hua, last2=Chen, first2=Ying, date=2016, title=Atomically Thin Boron Nitride: Unique Properties and Applications, journal=Advanced Functional Materials, volume=26, issue=16, pages=2594–2608, doi=10.1002/adfm.201504606, arxiv=1605.01136, s2cid=102038593 {{Cite book, doi=10.1016/bs.semsem.2016.04.004, isbn=978-0-12-804272-4, title=Semiconductors and Semimetals, last1=Bhimanapati, first1=G. R., last2=Glavin, first2=N. R., last3=Robinson, first3=J. A., date=2016-01-01, publisher=Elsevier, editor1-last= Iacopi , editor1-first= Francesca , editor-link1=Francesca Iacopi , editor2-last=Boeckl , editor2-first=John J. , editor3-last=Jagadish , editor3-first= Chennupati , series=2D Materials, volume=95, pages=101–147 {{Cite journal, last1=Wang, first1=Zifeng, last2=Tang, first2=Zijie, last3=Xue, first3=Qi, last4=Huang, first4=Yan, last5=Huang, first5=Yang, last6=Zhu, first6=Minshen, last7=Pei, first7=Zengxia, last8=Li, first8=Hongfei, last9=Jiang, first9=Hongbo, date=2016, title=Fabrication of Boron Nitride Nanosheets by Exfoliation, journal=The Chemical Record, volume=16, issue=3, pages=1204–1215, doi=10.1002/tcr.201500302, pmid=27062213 {{Cite journal, last1=Zhi, first1=Chunyi, last2=Bando, first2=Yoshio, last3=Tang, first3=Chengchun, last4=Kuwahara, first4=Hiroaki, last5=Golberg, first5=Dimitri, date=2009, title=Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties, journal=Advanced Materials, volume=21, issue=28, pages=2889–2893, doi=10.1002/adma.200900323, s2cid=95785929 Two-dimensional nanomaterials Boron compounds Boron–nitrogen compounds