|
Block Copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylon 6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid, which give nylon 66 its name. Aside from its superior physical characteristics, nylon 66 is attractive because its precursors are inexpensive. Synthesis and manufacturing Hexamethylenediamine (top) and adipic acid (bottom), monomers used for polycondensation of Nylon 66. Nylon 66 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined in water. In the original implementation, the resulting ammonium/ carboxylate salt was isolated and then heated either in batches or continuously to induce polycondensation.n(HOOC - (CH2)4 - COOH) + n(H2N - (CH2)6 - NH2) -> OC - (CH2)4 - CO - NH - (CH2)6 - NH - n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Definition For A Block In Polymer Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mayo–Lewis Equation
The Mayo–Lewis equation or copolymer equation in polymer chemistry describes the distribution of monomers in a copolymer. It was proposed by Frank R. Mayo and Frederick M. Lewis.''Copolymerization. I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate'' Frank R. Mayo and Frederick M. Lewis J. Am. Chem. Soc.; 1944; 66(9) pp 1594 - 1601; The equation considers a monomer mix of two components M_1\, and M_2\, and the four different reactions that can take place at the reactive chain end terminating in either monomer (M_1^*\, and M_2^*\,) with their reaction rate constants k\,: :M_1^* + M_1 \xrightarrow M_1M_1^* \, :M_1^* + M_2 \xrightarrow M_1M_2^* \, :M_2^* + M_2 \xrightarrow M_2M_2^* \, :M_2^* + M_1 \xrightarrow M_2M_1^* \, The reactivity ratio for each propagating chain end is defined as the ratio of the rate constant for addition of a monomer of the species already at the chain end to the rate constant for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
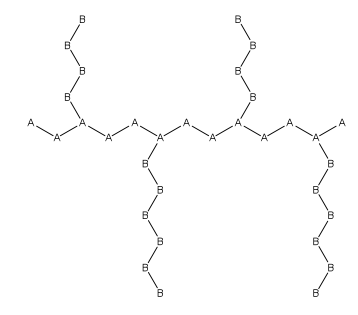
Graft Polymer
In polymer chemistry, graft polymers are segmented copolymers with a linear backbone of one composite and randomly distributed branches of another composite. The picture labeled "graft polymer" shows how grafted chains of species B are covalently bonded to polymer species A. Although the side chains are structurally distinct from the main chain, the individual grafted chains may be homopolymers or copolymers. Graft polymers have been synthesized for many decades and are especially used as impact resistant materials, thermoplastic elastomers, compatibilizers, or emulsifiers for the preparation of stable blends or alloys. One of the better-known examples of a graft polymer is a component used in high impact polystyrene, consisting of a polystyrene backbone with polybutadiene grafted chains. General properties Graft copolymer In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group (side group) has a different definition. Conventions The placeholder R is often used as a generic placeholder for alkyl (saturated hydrocarbon) group side chains in chemical structure diagrams. To indicate other non-carbon groups in structure diagrams, X, Y, or Z are often used. History The ''R'' symbol was introduced by 19th-century French chemist Charles Frédéric Gerhardt, who advocated its adoption on the grounds that it would be widely recognizable and intelligible given its correspondence in multiple European languages to the initial letter of "root" or "residue": ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Branching (polymer Chemistry)
In polymer chemistry, branching is the regular or irregular attachment of side chains to a polymer's backbone chain. It occurs by the replacement of a substituent (e.g. a hydrogen atom) on a monomer subunit by another covalently-bonded chain of that polymer; or, in the case of a graft copolymer, by a chain of another type. Branched polymers have more compact and symmetrical molecular conformations, and exhibit intra-heterogeneous dynamical behavior with respect to the unbranched polymers. In crosslinking rubber by vulcanization, short sulfur branches link polyisoprene chains (or a synthetic variant) into a multiple-branched thermosetting elastomer. Rubber can also be so completely vulcanized that it becomes a rigid solid, so hard it can be used as the bit in a smoking pipe. Polycarbonate chains can be crosslinked to form the hardest, most impact-resistant thermosetting plastic, used in safety glasses. Branching may result from the formation of carbon-carbon or various ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Backbone Chain
In polymer science, the polymer chain or simply backbone of a polymer is the main chain of a polymer. Polymers are often classified according to the elements in the main chains. The character of the backbone, i.e. its flexibility, determines the properties of the polymer (such as the glass transition temperature). For example, in polysiloxanes (silicone), the backbone chain is very flexible, which results in a very low glass transition temperature of . The polymers with rigid backbones are prone to crystallization (e.g. polythiophenes) in thin films and in solution. Crystallization in its turn affects the optical properties of the polymers, its optical band gap and electronic levels. Organic polymers : Common synthetic polymers have main chains composed of carbon, i.e. C-C-C-C.... Examples include polyolefins such as polyethylene ((CH2CH2)n) and many substituted derivative ((CH2CH(R))n) such as polystyrene (R = C6H5), polypropylene (R = CH3), and acrylates (R = CO2R'). Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |