block copolymers on:
[Wikipedia]
[Google]
[Amazon]
In
 Block copolymers comprise two or more
Block copolymers comprise two or more
 In stereoblock copolymers the blocks or units differ only in the
In stereoblock copolymers the blocks or units differ only in the
 Graft copolymers are a special type of branched copolymer wherein the side chains are structurally distinct from the main chain. Typically, the main chain is formed from one type of monomer (A) and branches are formed from another monomer (B), or the side-chains have constitutional or configurational features that differ from those in the main chain.
The individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example,
Graft copolymers are a special type of branched copolymer wherein the side chains are structurally distinct from the main chain. Typically, the main chain is formed from one type of monomer (A) and branches are formed from another monomer (B), or the side-chains have constitutional or configurational features that differ from those in the main chain.
The individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example,
 Star copolymers have several polymer chains connected to a central core.
Star copolymers have several polymer chains connected to a central core.
 Block copolymers can "microphase separate" to form periodic
Block copolymers can "microphase separate" to form periodic
Introduction to Polymer Chemistry
Polymer chemistry
polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
, a copolymer is a polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
derived from more than one species of monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
. The polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively.
Copolymers can be characterized by a variety of techniques such as NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
and size-exclusion chromatography
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecul ...
to determine the molecular size, weight, properties, and composition of the material.
Commercial copolymers include acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)''x''·(C4H6)''y''·(C3H3N)''z'' is a common thermoplastic polymer. Its glass transition temperature is approximately . ABS is amorphous and therefore has no true melting point.
A ...
(ABS), styrene/butadiene co-polymer
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging st ...
(SBR), nitrile rubber
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is ...
, styrene-acrylonitrile Styrene acrylonitrile resin is a copolymer plastic consisting of styrene and acrylonitrile. It is also known as SAN. It is widely used in place of polystyrene owing to its greater thermal resistance. The chains of between 70 and 80% by weight styren ...
, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate
Ethylene-vinyl acetate (EVA), also known as poly (ethylene-vinyl acetate) (PEVA), is the copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 40%, with the remainder being ethylene. There are thr ...
, all of which are formed by chain-growth polymerization
Chain-growth polymerization ( AE) or chain-growth polymerisation ( BE) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active ...
. Another production mechanism is step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurrin ...
, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the compa ...
and nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atom ...
, as well as the copolyester family. Copolymers can be used to develop commercial goods or drug delivery vehicles.
Since a copolymer consists of at least two types of constituent units (also structural unit
In polymer chemistry, a structural unit is a building block of a polymer chain. It is the result of a monomer which has been polymerized into a long chain.
There may be more than one structural unit in the repeat unit. When different monomers ar ...
s), copolymers can be classified based on how these units are arranged along the chain
A chain is a wikt:series#Noun, serial assembly of connected pieces, called links, typically made of metal, with an overall character similar to that of a rope in that it is flexible and curved in compression (physics), compression but line (g ...
. ''Linear copolymers'' consist of a single main chain
A ridge or a mountain ridge is a geographical feature consisting of a chain of mountains or hills that form a continuous elevated crest for an extended distance. The sides of the ridge slope away from the narrow top on either side. The line ...
and include alternating copolymers, statistical copolymers, and block copolymers. '' Branched copolymers'' consist of a single main chain with one or more polymeric side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
s, and can be grafted
Grafting or graftage is a horticultural technique whereby tissues of plants are joined so as to continue their growth together. The upper part of the combined plant is called the scion () while the lower part is called the rootstock. The succ ...
, star shaped, or have other architectures.
Reactivity ratios
The ''reactivity ratio'' of a growing copolymer chain terminating in a given monomer is the ratio of thereaction rate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the ...
for addition of the same monomer and the rate constant for addition of the other monomer. That is, and , where for example is the rate constant for propagation of a polymer chain ending in monomer 1 (or A) by addition of monomer 2 (or B).
The composition and structural type of the copolymer depend on these reactivity ratios r1 and r2 according to the Mayo–Lewis equation The Mayo–Lewis equation or copolymer equation in polymer chemistry describes the distribution of monomers in a copolymer. It was proposed by Frank R. Mayo and Frederick M. Lewis.''Copolymerization. I. A Basis for Comparing the Behavior of Monomer ...
, also called the copolymerization equation or copolymer equation, for the relative instantaneous rates of incorporation of the two monomers.
Linear copolymers
Block copolymers
 Block copolymers comprise two or more
Block copolymers comprise two or more homopolymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
subunits linked by covalent bonds. The union of the homopolymer subunits may require an intermediate non-repeating subunit, known as a junction block. Diblock copolymers have two distinct blocks; triblock copolymers have three. Technically, a block is a portion of a macromolecule, comprising many units, that has at least one feature which is not present in the adjacent portions. A possible sequence of repeat units A and B in a triblock copolymer might be ~A-A-A-A-A-A-A-B-B-B-B-B-B-B-A-A-A-A-A~.
Block copolymers are made up of blocks of different polymerized
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
. For example, polystyrene-b-poly(methyl methacrylate) or PS-b-PMMA (where b = block) is usually made by first polymerizing styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
, and then subsequently polymerizing methyl methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA) ...
(MMA) from the reactive end of the polystyrene chains. This polymer is a "diblock copolymer" because it contains two different chemical blocks. Triblocks, tetrablocks, multiblocks, etc. can also be made. Diblock copolymers are made using living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
techniques, such as atom transfer free radical polymerization ( ATRP), reversible addition fragmentation chain transfer (RAFT
A raft is any flat structure for support or transportation over water. It is usually of basic design, characterized by the absence of a hull. Rafts are usually kept afloat by using any combination of buoyant materials such as wood, sealed barrel ...
), ring-opening metathesis polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous an ...
(ROMP), and living cationic or living anionic polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
s. An emerging technique is chain shuttling polymerization Chain shuttling polymerization is a dual-catalyst method for producing block copolymers with alternating or variable tacticity. The desired effect of this method is to generate hybrid polymers that bear the properties of both polymer chains, such ...
.
The synthesis of block copolymers requires that both reactivity ratios are much larger than unity (r1 >> 1, r2 >> 1) under the reaction conditions, so that the terminal monomer unit of a growing chain tends to add a similar unit most of the time.
The "blockiness" of a copolymer is a measure of the adjacency of comonomers vs their statistical distribution. Many or even most synthetic polymers are in fact copolymers, containing about 1-20% of a minority monomer. In such cases, blockiness is undesirable. A ''block index'' has been proposed as a quantitative measure of blockiness or deviation from random monomer composition.
Alternating copolymers
An alternating copolymer has regular alternating A and B units, and is often described by the formula: -A-B-A-B-A-B-A-B-A-B-, or -(-A-B-)n-. The molar ratio of each monomer in the polymer is normally close to one, which happens when the reactivity ratios r1 and r2 are close to zero, as can be seen from the Mayo–Lewis equation. For example, in the free-radical copolymerization ofstyrene maleic anhydride
Styrene maleic anhydride (SMA or SMAnh) is a synthetic polymer that is built-up of styrene and maleic anhydride monomers. The monomers can be almost perfectly alternating, making it an alternating copolymer, but (random) copolymerisation with less ...
copolymer, r1 = 0.097 and r2 = 0.001, so that most chains ending in styrene add a maleic anhydride unit, and almost all chains ending in maleic anhydride add a styrene unit. This leads to a predominantly alternating structure.
A step-growth copolymer -(-A-A-B-B-)n- formed by the condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of two bifunctional
In organic chemistry, when a single organic molecule has two different functional groups, it is called a bifunctional molecule . A bifunctional molecule has the properties of two different types of functional groups, such as an alcohol (), amide ( ...
monomers A–A and B–B is in principle a perfectly alternating copolymer of these two monomers, but is usually considered as a homopolymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
of the dimeric repeat unit A-A-B-B. An example is nylon 66
Nylon 66 (loosely written nylon 6-6, nylon 6/6, nylon 6,6, or nylon 6:6) is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atom ...
with repeat unit -OC-( CH2)4-CO-NH-(CH2)6-NH-, formed from a dicarboxylic acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show ...
monomer and a diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive.
In terms of quantiti ...
monomer.
Periodic copolymers
Periodic copolymers have units arranged in a repeating sequence. For two monomers A and B, for example, they might form the repeated pattern (A-B-A-B-B-A-A-A-A-B-B-B)n.Statistical copolymers
In statistical copolymers the sequence of monomer residues follows a statistical rule. If the probability of finding a given type monomer residue at a particular point in the chain is equal to the mole fraction of that monomer residue in the chain, then the polymer may be referred to as a truly random copolymerPainter P. C. and Coleman M. M., ''Fundamentals of Polymer Science'', CRC Press, 1997, p 14. (structure 3). Statistical copolymers are dictated by the reaction kinetics of the two chemically distinct monomer reactants, and are commonly referred to interchangeably as “random” in the polymer literature.Chanda, M. ''Introduction to Polymer Science and Chemistry''. Second Edition. CRC Press, 2013. As with other types of copolymers, random copolymers can have interesting and commercially desirable properties that blend those of the individual homopolymers. Examples of commercially relevant random copolymers includerubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, a ...
s made from styrene-butadiene copolymers and resins from styrene-acrylic or methacrylic acid
Methacrylic acid, abbreviated MAA, is an organic compound. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced indus ...
derivatives. Copolymerization is particularly useful in tuning the glass transition
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
temperature, which is important in the operating conditions of polymers; it is assumed that each monomer occupies the same amount of free volume whether it is in a copolymer or homopolymer, so the glass transition
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
temperature (Tg) falls between the values for each homopolymer and is dictated by the mole or mass fraction of each component.
A number of parameters are relevant in the composition of the polymer product; namely, one must consider the reactivity ratio of each component. Reactivity ratios describe whether the monomer reacts preferentially with a segment of the same type or of the other type. For example, a reactivity ratio that is less than one for component 1 indicates that this component reacts with the other type of monomer more readily. Given this information, which is available for a multitude of monomer combinations in the “Wiley Database of Polymer Properties”, the Mayo-Lewis equation can be used to predict the composition of the polymer product for all initial mole fractions of monomer. This equation is derived using the Markov model
In probability theory, a Markov model is a stochastic model used to model pseudo-randomly changing systems. It is assumed that future states depend only on the current state, not on the events that occurred before it (that is, it assumes the Mark ...
, which only considers the last segment added as affecting the kinetics of the next addition; the Penultimate Model considers the second-to-last segment as well, but is more complicated than is required for most systems. When both reactivity ratios are less than one, there is an azeotropic point in the Mayo-Lewis plot. At this point, the mole fraction of monomer equals the composition of the component in the polymer.
There are several ways to synthesize random copolymers. The most common synthesis method is free radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechan ...
; this is especially useful when the desired properties rely on the composition of the copolymer rather than the molecular weight, since free radical polymerization produces relatively disperse polymer chains. Free radical polymerization is less expensive than other methods, and produces high-molecular weight polymer quickly. Several methods offer better control over dispersity
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an incons ...
. Anionic polymerization can be used to create random copolymers, but with several caveats: if carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
s of the two components do not have the same stability, only one of the species will add to the other. Additionally, anionic polymerization is expensive and requires very clean reaction conditions, and is therefore difficult to implement on a large scale. Less disperse random copolymers are also synthesized by ″living″ controlled radical polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term " reversible-deactivation radical polymerization" instead of "liv ...
methods, such as atom-transfer radical-polymerization Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal ca ...
(ATRP), nitroxide mediated radical polymerization Nitroxide-mediated radical polymerization is a method of radical polymerization that makes use of an nitroxide initiator to generate polymers with well controlled stereochemistry and a very low dispersity. It is a type of reversible-deactivation rad ...
(NMP), or Reversible addition−fragmentation chain-transfer polymerization
Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound (or similar, fr ...
(RAFT). These methods are favored over anionic polymerization because they can be performed in conditions similar to free radical polymerization. The reactions require longer experimentation periods than free radical polymerization, but still achieve reasonable reaction rates.
Stereoblock copolymers
 In stereoblock copolymers the blocks or units differ only in the
In stereoblock copolymers the blocks or units differ only in the tacticity
Tacticity (from el, τακτικός, taktikos, "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical p ...
of the monomers.
Gradient copolymers
In gradient copolymers the monomer composition changes gradually along the chain.Branched copolymers
There are a variety of architectures possible for nonlinear copolymers. Beyond grafted and star polymers discussed below, other common types of branched copolymers include brush copolymers and comb copolymers.Graft copolymers
 Graft copolymers are a special type of branched copolymer wherein the side chains are structurally distinct from the main chain. Typically, the main chain is formed from one type of monomer (A) and branches are formed from another monomer (B), or the side-chains have constitutional or configurational features that differ from those in the main chain.
The individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example,
Graft copolymers are a special type of branched copolymer wherein the side chains are structurally distinct from the main chain. Typically, the main chain is formed from one type of monomer (A) and branches are formed from another monomer (B), or the side-chains have constitutional or configurational features that differ from those in the main chain.
The individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example, polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is ...
chains may be grafted onto polybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of ti ...
, a synthetic rubber
A synthetic rubber is an artificial elastomer. They are polymers synthesized from petroleum byproducts. About 32-million metric tons of rubbers are produced annually in the United States, and of that amount two thirds are synthetic. Synthetic rubbe ...
which retains one reactive C=C double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
per repeat unit
In polymer chemistry, a repeat unit or repeating unit (or mer) is a part of a polymer whose repetition would produce the complete polymer chain (except for the end-groups) by linking the repeat units together successively along the chain, like the ...
. The polybutadiene is dissolved in styrene, which is then subjected to free-radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanis ...
. The growing chains can add across the double bonds of rubber molecules forming polystyrene branches. The graft copolymer is formed in a mixture with ungrafted polystyrene chains and rubber molecules.
As with block copolymers, the quasi- composite product has properties of both "components." In the example cited, the rubbery chains absorb energy when the substance is hit, so it is much less brittle than ordinary polystyrene. The product is called high-impact polystyrene, or HIPS.
Star copolymers
 Star copolymers have several polymer chains connected to a central core.
Star copolymers have several polymer chains connected to a central core.
Microphase separation
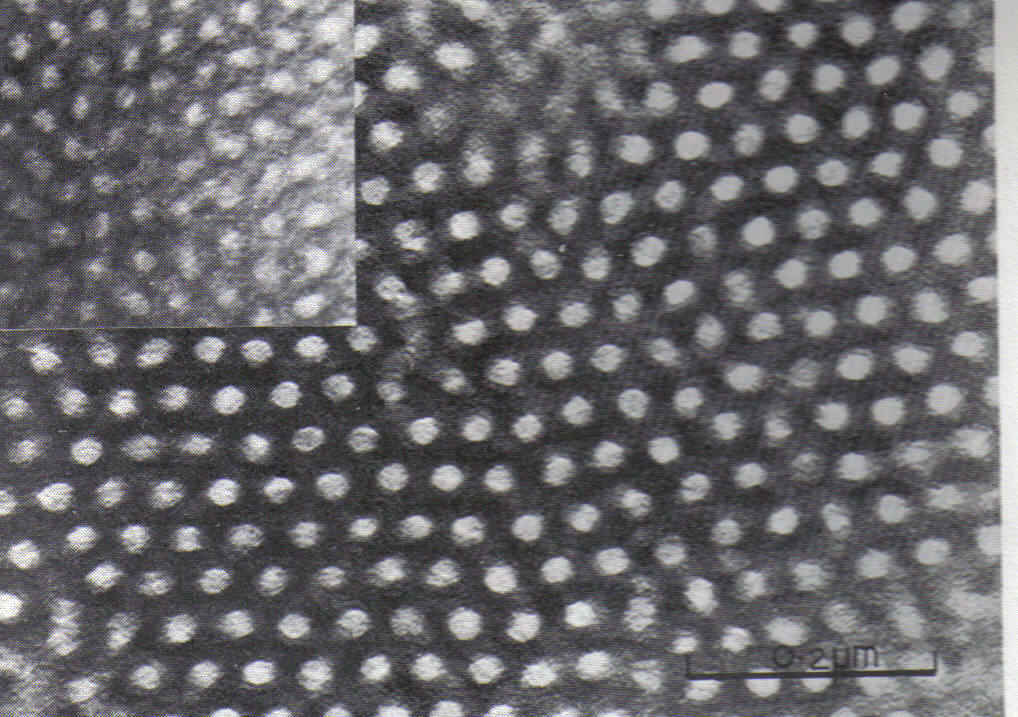
 Block copolymers can "microphase separate" to form periodic
Block copolymers can "microphase separate" to form periodic nanostructures
A nanostructure is a structure of intermediate size between microscopic and molecular structures. Nanostructural detail is microstructure at nanoscale.
In describing nanostructures, it is necessary to differentiate between the number of dime ...
, such as styrene-butadiene-styrene block copolymer. The polymer is known as Kraton and is used for shoe soles and adhesives. Owing to the microfine structure, transmission electron microscope or TEM was used to examine the structure. The butadiene matrix was stained with osmium tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the ...
to provide contrast in the image. The material was made by living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
so that the blocks are almost monodisperse to create a regular microstructure. The molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
of the polystyrene blocks in the main picture is 102,000; the inset picture has a molecular weight of 91,000, producing slightly smaller domains.
Microphase separation is a situation similar to that of oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturate ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. Oil and water are immiscible (i.e., they can phase separate). Due to the incompatibility between the blocks, block copolymers undergo a similar phase separation. Since the blocks are covalently bonded to each other, they cannot demix macroscopically like water and oil. In "microphase separation," the blocks form nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, ...
-sized structures. Depending on the relative lengths of each block, several morphologies can be obtained. In diblock copolymers, sufficiently different block lengths lead to nanometer-sized spheres of one block in a matrix of the second (e.g., PMMA PMMA may refer to:
* para-Methoxymethamphetamine
''para''-Methoxy-''N''-methylamphetamine (also known as PMMA, Red Mitsubishi), chemically known as methyl-MA, 4-methoxy-''N''-methylamphetamine, 4-MMA) or (4-PMDA, as listed to its original phy ...
in polystyrene). Using less different block lengths, a "hexagonally packed cylinder" geometry can be obtained. Blocks of similar length form layers (often called lamellae
Lamella (plural lamellae) means a small plate or flake in Latin, and in English may refer to:
Biology
* Lamella (mycology), a papery rib beneath a mushroom cap
* Lamella (botany)
* Lamella (surface anatomy), a plate-like structure in an animal
* ...
in the technical literature). Between the cylindrical and lamellar phase is the gyroid
A gyroid is an infinitely connected triply periodic minimal surface discovered by Alan Schoen in 1970.
History and properties
The gyroid is the unique non-trivial embedded member of the associate family of the Schwarz P and D surfaces. It ...
phase. The nanoscale structures created from block copolymers can potentially be used to create devices for computer memory
Memory is the faculty of the mind by which data or information is encoded, stored, and retrieved when needed. It is the retention of information over time for the purpose of influencing future action. If past events could not be remembered ...
, nanoscale-templating, and nanoscale separations. Block copolymers are sometimes used as a replacement for phospholipids in model lipid bilayer
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in ...
s and liposome
A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delive ...
s for their superior stability and tunability.
Polymer scientists use thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws o ...
to describe how the different blocks interact. The product of the degree of polymerization, ''n'', and the Flory-Huggins interaction parameter
Interaction is action that occurs between two or more objects, with broad use in philosophy and the sciences. It may refer to:
Science
* Interaction hypothesis, a theory of second language acquisition
* Interaction (statistics)
* Interactions o ...
, , gives an indication of how incompatible the two blocks are and whether they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product is greater than 10.5. If is less than 10.5, the blocks will mix and microphase separation is not observed. The incompatibility between the blocks also affects the solution behavior of these copolymers and their adsorption behavior on various surfaces.
Block copolymers are able to self-assemble in selective solvents to form micelles among other structures.
In thin films, block copolymers are of great interest as masks in the lithographic patterning of semiconductor materials for applications in high density data storage. A key challenge is to minimise the feature size and much research is in progress on this.
Characterization
Characterization
Characterization or characterisation is the representation of persons (or other beings or creatures) in narrative and dramatic works. The term character development is sometimes used as a synonym. This representation may include direct methods ...
techniques for copolymers are similar to those for other polymeric materials. These techniques can be used to determine the average molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
, molecular size, chemical composition, molecular homogeneity
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, ...
, and physiochemical properties of the material. However, given that copolymers are made of base polymer components with heterogenous properties, this may require multiple characterization techniques to accurately characterize these copolymers.
Spectroscopic techniques, such as nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
, infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, and UV spectroscopy
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
, are often used to identify the molecular structure and chemical composition of copolymers. In particular, NMR can indicate the tacticity
Tacticity (from el, τακτικός, taktikos, "relating to arrangement or order") is the relative stereochemistry of adjacent chiral centers within a macromolecule. The practical significance of tacticity rests on the effects on the physical p ...
and configuration of polymeric chains while IR can identify functional groups attached to the copolymer.
Scattering techniques, such as static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight ''Mw'' of a macromolecule like a polymer or a protein in solution. Measurement of the scattering ...
, dynamic light scattering
Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. In the scope of DLS, temporal fluctuations are usually analyzed usin ...
, and small-angle neutron scattering
Small-angle neutron scattering (SANS) is an experimental technique that uses elastic neutron scattering at small scattering angles to investigate the structure of various substances at a mesoscopic scale of about 1–100 nm.
Small angle ...
, can determine the molecular size and weight of the synthesized copolymer. Static light scattering and dynamic light scattering use light to determine the average molecular weight and behavior of the copolymer in solution whereas small-angle neutron scattering uses neutrons to determine the molecular weight and chain length.
Differential scanning calorimetry is a thermoanalytical technique used to determine the thermal events of the copolymer as a function of temperature. It can indicate when the copolymer is undergoing a phase transition, such as crystallization or melting, by measuring the heat flow required to maintain the material and a reference at a constantly increasing temperature.
Thermogravimetric analysis
Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of a sample is measured over time as the temperature changes. This measurement provides information about physical phenomena, such ...
is another thermoanalytical technique used to access the thermal stability of the copolymer as a function of temperature. This provides information on any changes to the physicochemical properties, such as phase transitions, thermal decompositions, and redox reactions.
Size-exclusion chromatography
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecul ...
can separate copolymers with different molecular weights based on their hydrodynamic volume. From there, the molecular weight can be determined by deriving the relationship from its hydrodynamic volume. Larger copolymers tend to elute first as they do not interact with the column as much. The collected material is commonly detected by light scattering methods, a refractometer, or a viscometer to determine the concentration of the eluted copolymer.
Applications
Block copolymers
A common application of block copolymers is to developthermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric propertie ...
s (TPEs). Early commercial TPEs were developed from polyurethranes (TPUs), consisting of alternating soft segments and hard segments, and are used in automative bumpers and snowmobile treads. Styrenic TPEs entered the market later, and are used in footwear, bitumen modification, thermoplastic blending, adhesives, and cable insulation and gaskets. Modifying the linkages between the blocks resulted in newer TPEs based on polyesters (TPES) and polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
s (TPAs), used in hose tubing, sport goods, and automative components.
Amphiphilic
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compoun ...
block copolymers have the ability to form micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated col ...
s and nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
s. Due to this property, amphiphilic block copolymers have garnered much attention in research on vehicles for drug delivery. Similarly, amphiphilic block copolymers can be used for the removal of organic contaminants from water either through micelle formation or film preparation.
Alternating copolymers
The styrene-maleic acid (SMA) alternating copolymer displays amphiphilicity depending on pH, allowing it to change conformations in different environments. Some conformations that SMA can take are random coil formation, compact globular formation, micelles, and nanodiscs. SMA has been used as a dispersing agent for dyes and inks, as drug delivery vehicles, and for membrane solubilization.Copolymer engineering
Copolymerization is used to modify the properties of manufactured plastics to meet specific needs, for example to reduce crystallinity, modifyglass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
, control wetting properties or to improve solubility. It is a way of improving mechanical properties, in a technique known as rubber toughening Rubber toughening is a process in which rubber nanoparticles are interspersed within a polymer matrix to increase the mechanical robustness, or toughness, of the material. By "toughening" a polymer it is meant that the ability of the polymeric subst ...
. Elastomeric phases within a rigid matrix act as crack arrestors, and so increase the energy absorption when the material is impacted for example. Acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)''x''·(C4H6)''y''·(C3H3N)''z'' is a common thermoplastic polymer. Its glass transition temperature is approximately . ABS is amorphous and therefore has no true melting point.
A ...
is a common example.
See also
* Copolymers section of Polymer article *Thermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric propertie ...
* Tholin
Tholins (after the Greek (') "hazy" or "muddy"; from the ancient Greek word meaning "sepia ink") are a wide variety of organic compounds formed by solar ultraviolet or cosmic ray irradiation of simple carbon-containing compounds such as carbon ...
References
External links
{{Commons, CopolymersIntroduction to Polymer Chemistry
Polymer chemistry