|
Bismuth Oxide
Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite (tetragonal, much more rare), but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the " Dragon's eggs" effect in fireworks, as a replacement of red lead. Structure The structures adopted by differ substantially from those of arsenic(III) oxide, , and antimony(III) oxide, .Wells, A.F. (1984) ''Structural Inorganic Chemistry''. 5th. London, England: Oxford University Press. p.890 Bismuth oxide, has five crystallographic polymorphs. The room temperature phase, α- has a monoclinic crystal structure. There are three high temperature phases, a tetragonal β-phase, a body-centred cubic γ-phase, a cubic δ- phase and an ε-phase. The room temperature α-phase has a complex structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismite
Bismite is a bismuth oxide mineral, bismuth trioxide or Bi2O3. It is a monoclinic mineral, but the typical form of occurrence is massive and clay-like with no macroscopic crystals. The color varies from green to yellow. It has a Mohs hardness of 4 to 5 and a specific gravity of 8.5 to 9.5, quite high for a nonmetallic mineral. Bismite is a secondary oxidation zone mineral which forms from primary bismuth minerals. It was first described from Goldfield, Nevada in 1868, and later from the Schneeberg District, Ore Mountains, Saxony, Germany. See also Bismuth trioxide Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite (tetragonal, much more rare) ... — ''details on the chemistry of this substance.'' References Mindat localities [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Lead
Red is the color at the long wavelength end of the visible spectrum of light, next to orange and opposite violet. It has a dominant wavelength of approximately 625–740 nanometres. It is a primary color in the RGB color model and a secondary color (made from magenta and yellow) in the CMYK color model, and is the complementary color of cyan. Reds range from the brilliant yellow-tinged scarlet and vermillion to bluish-red crimson, and vary in shade from the pale red pink to the dark red burgundy. Red pigment made from ochre was one of the first colors used in prehistoric art. The Ancient Egyptians and Mayans colored their faces red in ceremonies; Roman generals had their bodies colored red to celebrate victories. It was also an important color in China, where it was used to color early pottery and later the gates and walls of palaces. In the Renaissance, the brilliant red costumes for the nobility and wealthy were dyed with kermes and cochineal. The 19th century brought ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacancy Defect
In crystallography, a vacancy is a type of point defect in a crystal where an atom is missing from one of the lattice sites.Ehrhart, P. (1991) "Properties and interactions of atomic defects in metals and alloys", chapter 2, p. 88 in ''Landolt-Börnstein, New Series III'', Vol. 25, Springer, Berlin Crystals inherently possess imperfections, sometimes referred to as crystalline defects. Vacancies occur naturally in all crystalline materials. At any given temperature, up to the melting point of the material, there is an equilibrium concentration (ratio of vacant lattice sites to those containing atoms). At the melting point of some metals the ratio can be approximately 1:1000. This temperature dependence can be modelled by :N_ = N \exp(-Q_/k_ T) where is the vacancy concentration, is the energy required for vacancy formation, is the Boltzmann constant, is the absolute temperature, and is the concentration of atomic sites i.e. : N = m N_ / M where is mass, Avogadro con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Matter
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth Silicon Oxide
Bismuth silicon oxide is a solid inorganic compound of bismuth, silicon and oxygen. Its most common chemical formula is Bi12SiO20, though other compositions are also known. It occurs naturally as the mineral sillénite and can be produced synthetically, by heating a mixture of bismuth and silicon oxides. Centimeter-sized single crystals of Bi12SiO20 can be grown by the Czochralski process from the molten phase. They exhibit piezoelectric, electro-optic, elasto-optic, photorefractive and photoconductive properties, and therefore have potential applications in spatial light modulators, acoustic delay lines and hologram recording equipment. Bi12SiO20 can be obtained as a whitish powder with band gap of approximately 3.2 eV starting from bismuth subcarbonate and silica in presence of ethyleneglycol. 29Si solid-state NMR is used to proof that the Si(IV) cations are sharing oxygen atoms with the Bi(III) cations. The 29Si chemical shift (δ) in Bi12SiO20 is −78.1 ppm. Unlike the bismu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
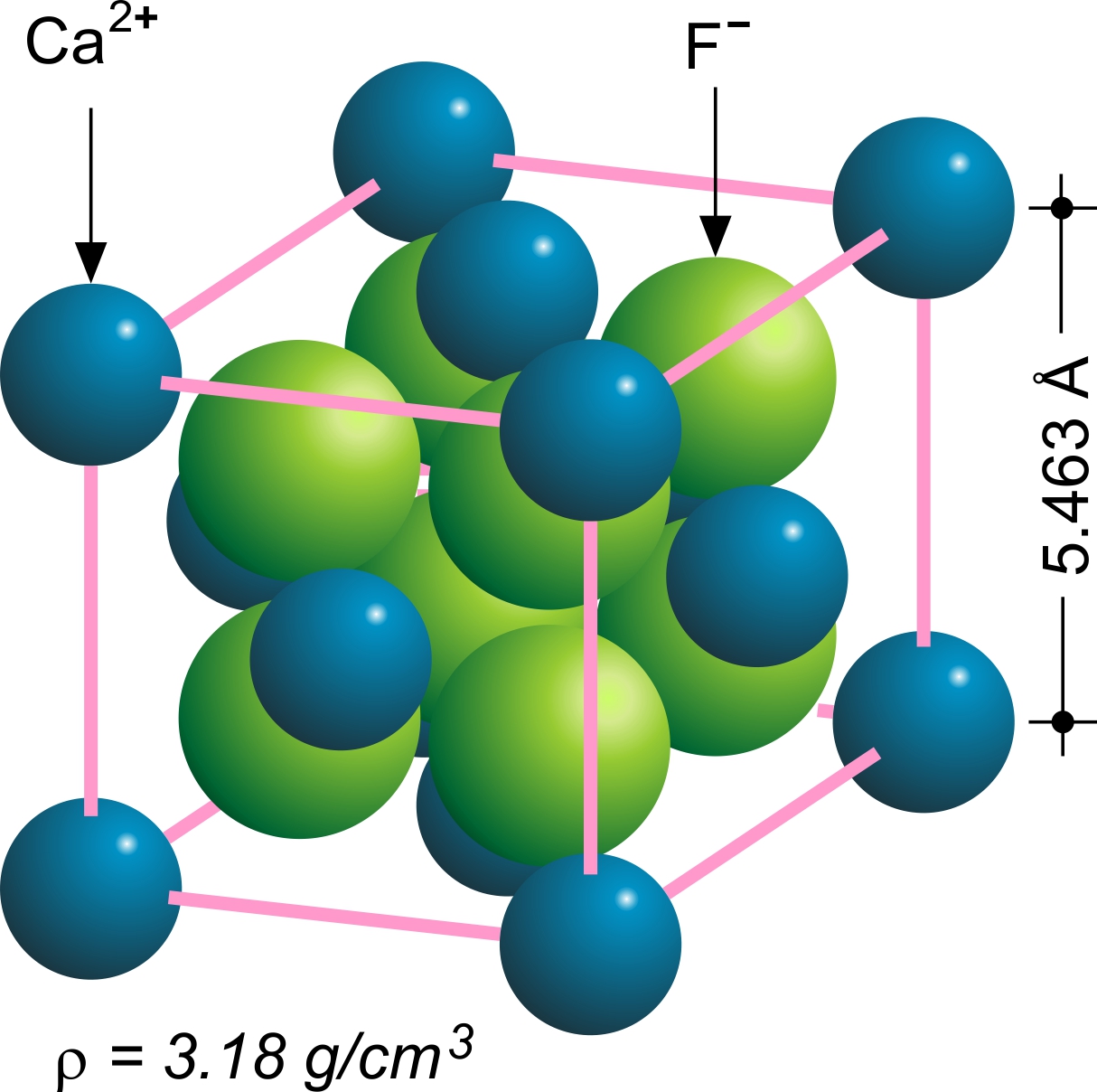
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Body-centred Cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |