|
Bis(trifluoromethyl)peroxide
Bis(trifluoromethyl)peroxide (BTP) is a fluorocarbon derivative first produced by Frédéric Swarts. It has recently been discovered that it is a good initiator for the polymerization of unsaturated ethylene-like molecules. It produces good quality polymers which are quite stable. This property is the reason an economical synthesis is sought for BTP. This chemical is unusual in the fact that unlike many peroxides, bis(trifluoromethyl)peroxide is a gas, is nonexplosive and has good thermal stability.Ellingboe, E.K.; McCleiland A.L. Polymerization initiator. US 3202718, June 20, 1960 History Bis(trifluoromethyl)peroxide was first created in trace elements by an electrolysis reaction using aqueous solutions containing trifluoroacetate ion. This was one of the byproducts Frédéric Swarts got when performing trifluoromethylation reactions. Later it was discovered that Bis(trifluoromethyl)peroxide had some unusual properties. This began a search for a more economical production of Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Highly Toxic Gases
Many gases have toxic properties, which are often assessed using the LC50 (median lethal dose) measure. In the United States, many of these gases have been assigned an NFPA 704 health rating of 4 (may be fatal) or 3 (may cause serious or permanent injury), and/or exposure limits ( TLV, TWA or STEL) determined by the ACGIH professional association. Some, but by no means all, toxic gases are detectable by odor, which can serve as a warning. Among the best known toxic gases are carbon monoxide, chlorine, nitrogen dioxide and phosgene. Definition *Toxic: it is a chemical that has a median lethal concentration (LC50) in air of more than 200 parts per million (ppm) but not more than 2,000 parts per million by volume of gas or vapor, or more than 2 milligrams per liter but not more than 20 milligrams per liter of mist, fume or dust, when administered by continuous inhalation for 1 hour (or less if death occurs within 1 hour) to albino rats weighing between 200 and 300 grams each. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, i.e., they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its strength is a resu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frédéric Swarts
Frédéric Swarts (2 September 1866 – 6 September 1940) was a Belgian chemist who prepared the first chlorofluorocarbon, CF2Cl2 (Freon-12) as well as several other related compounds. He was a professor in the civil engineering at the University of Ghent. In addition to his work on organofluorine chemistry, he authored the textbook "Cours de Chimie Organique."Frédéric Swarts "Cours de Chimie Organique" Librairie Scientifique, A. Hermann (Paris), 1908. He was a son of Theodore Swarts (chemist, *1839 Antwerpen; †1911 Kortenberg, Belgium) and a colleague of Leo Baekeland Leo Hendrik Baekeland (November 14, 1863 – February 23, 1944) was a Belgian chemist. He is best known for the inventions of Velox photographic paper in 1893, and Bakelite in 1907. He has been called "The Father of the Plastics Industry" .... References * {{DEFAULTSORT:Swarts, Frederic 20th-century Belgian inventors Belgian chemists 1866 births 1940 deaths Academic staff of Ghent Universit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipinski's Rule Of Five
Lipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules. The rule describes molecular properties important for a drug's pharmacokinetics in the human body, including their absorption, distribution, metabolism, and excretion ("ADME"). However, the rule does not predict if a compound is pharmacologically active. The rule is important to keep in mind during drug discovery when a pharmacologically active lead structure is optimized step-wise to increase the activity and selectivity of the compound as well as to ensure drug-like phy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(trifluoromethyl) Disulfide
Bis(trifluoromethyl) disulfide (TFD) is a fluorinated organosulfur compound that was used as a fumigant. It is also an intermediate in the synthesis of triflic acid. It is a volatile liquid that is extremely toxic by inhalation. Synthesis TFD can be produced by reaction of perchloromethyl mercaptan or thiophosgene with sodium fluoride. Toxicity TFD is extremely toxic by inhalation. TFD is a powerful pulmonary agent that can cause severe pulmonary edema. TFD is about half as toxic as perfluoroisobutene. See also *Dimethyl(trifluoromethylthio)arsine * Perchloromethyl mercaptan *Thiophosgene *Perfluoroisobutene *Phosgene Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ... Reference Organic disulfides Trifluoromethylthio compounds Pulmonary agents Fumigants {{Organohalid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Peroxides
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form (the dot represents an unpaired electron). Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents. Types of organic peroxides Tert-Butyl hydroperoxide Structural Formula V2.svg, ''tert''-Butyl hydroperoxide, a hydroperoxide (formula: ROOH) that is used to epoxide alkenes. Dicumyl peroxide.svg, Dicumyl peroxide, a dialkyl peroxide (formula: ROOR) that is used to initiate po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
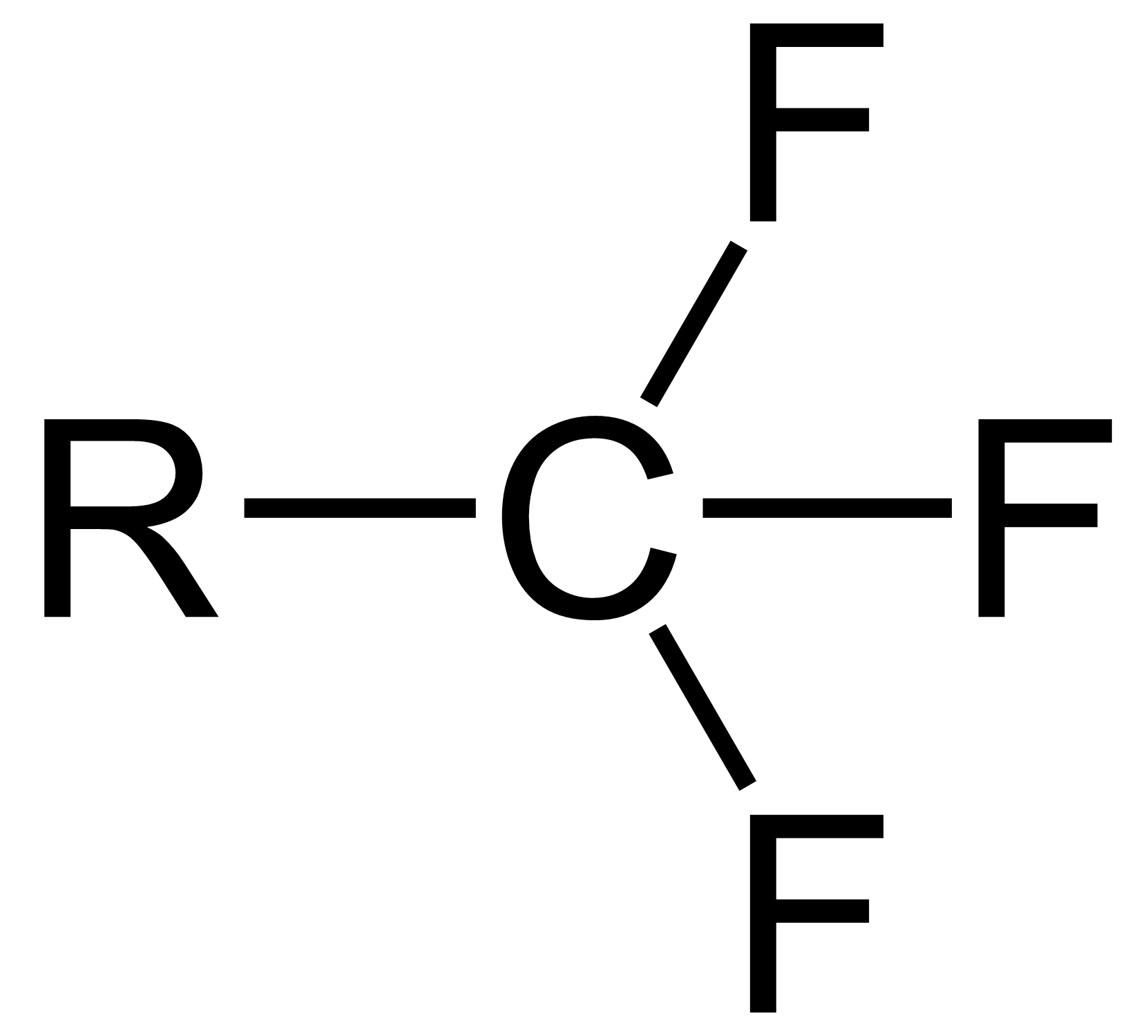
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |