|
Biological Photovoltaics
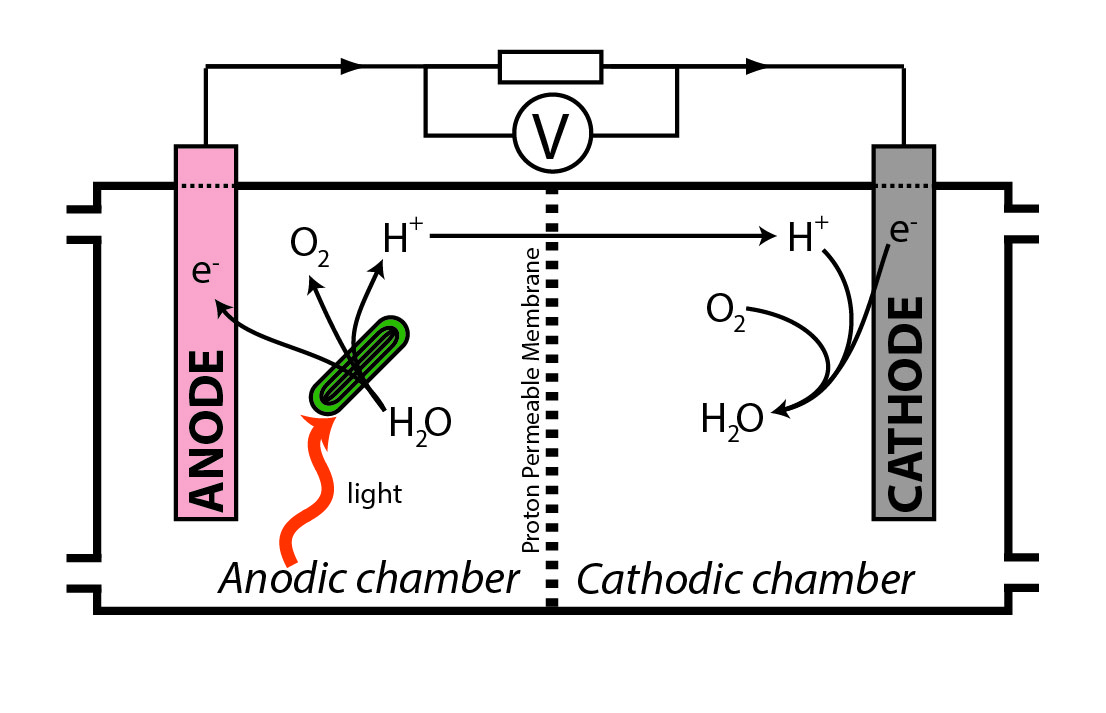
Biological photovoltaics, also called biophotovoltaics or BPV, is an energy-generating technology which uses oxygenic photoautotrophic organisms, or fractions thereof, to harvest light energy and produce electrical power. Biological photovoltaic devices are a type of biological electrochemical system, or microbial fuel cell, and are sometimes also called photo-microbial fuel cells or “living solar cells”. In a biological photovoltaic system, electrons generated by photolysis of water are transferred to an anode. A relatively high-potential reaction takes place at the cathode, and the resulting potential difference drives current through an external circuit to do useful work. It is hoped that using a living organism (which is capable of self-assembly and self-repair) as the light harvesting material, will make biological photovoltaics a cost-effective alternative to synthetic light-energy-transduction technologies such as silicon-based photovoltaics. Principle of operation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoautotrophic
Photoautotrophs are organisms that use light energy and inorganic carbon to produce organic materials. Eukaryotic photoautotrophs absorb energy through the chlorophyll molecules in their chloroplasts while prokaryotic photoautotrophs use chlorophylls and bacteriochlorophylls present in their cytoplasm. All known photoautotrophs perform photosynthesis. Examples include plants, algae, and cyanobacteria. Origin and the Great Oxidation Event Chemical and geological evidence indicate that photosynthetic cyanobacteria existed about 2.6 billion years ago and anoxygenic photosynthesis had been taking place since a billion years before that. Oxygenic photosynthesis was the primary source of oxygenation and led to the Great Oxidation Event (the Oxygen Catastrophe) roughly 2.4 to 2.1 billion years ago. Although the end of the Great Oxidation Event was marked by a significant decrease in gross primary productivity that eclipsed extinction events, the development of aerobic respiration enabled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Grid
An electrical grid is an interconnected network for electricity delivery from producers to consumers. Electrical grids vary in size and can cover whole countries or continents. It consists of:Kaplan, S. M. (2009). Smart Grid. Electrical Power Transmission: Background and Policy Issues. The Capital.Net, Government Series. Pp. 1-42. * power stations: often located near energy and away from heavily populated areas * electrical substations to step voltage up or down * electric power transmission to carry power long distances * electric power distribution to individual customers, where voltage is stepped down again to the required service voltage(s). Grids are nearly always synchronous, meaning all distribution areas operate with three phase alternating current (AC) frequencies synchronized (so that voltage swings occur at almost the same time). This allows transmission of AC power throughout the area, connecting a large number of electricity generators and consumers and potenti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Sericytochromatia, the proposed name of the paraphyletic and most basal group, is the ancestor of both the non-photosynthetic group Melainabacteria and the photosynthetic cyanobacteria, also called Oxyphotobacteria. Cyanobacteria use photosynthetic pigments, such as carotenoids, phycobilins, and various forms of chlorophyll, which absorb energy from light. Unlike heterotrophic prokaryotes, cyanobacteria have internal membranes. These are flattened sacs called thylakoids where photosynthesis is performed. Phototrophic eukaryotes such as green plants perform photosynthesis in plast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outer Mitochondrial Membrane
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organism, '' Henn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Insulator
An electrical insulator is a material in which electric current does not flow freely. The atoms of the insulator have tightly bound electrons which cannot readily move. Other materials—semiconductors and conductors—conduct electric current more easily. The property that distinguishes an insulator is its resistivity; insulators have higher resistivity than semiconductors or conductors. The most common examples are non-metals. A perfect insulator does not exist because even insulators contain small numbers of mobile charges (charge carriers) which can carry current. In addition, all insulators become electrically conductive when a sufficiently large voltage is applied that the electric field tears electrons away from the atoms. This is known as the breakdown voltage of an insulator. Some materials such as glass, paper and PTFE, which have high resistivity, are very good electrical insulators. A much larger class of materials, even though they may have lower bulk resistivity, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planktonic Biological Photovoltaic Device, Front And Rear Views
Plankton are the diverse collection of organisms found in water (or air) that are unable to propel themselves against a current (or wind). The individual organisms constituting plankton are called plankters. In the ocean, they provide a crucial source of food to many small and large aquatic organisms, such as bivalves, fish and whales. Marine plankton include bacteria, archaea, algae, protozoa and drifting or floating animals that inhabit the saltwater of oceans and the brackish waters of estuaries. Freshwater plankton are similar to marine plankton, but are found in the freshwaters of lakes and rivers. Plankton are usually thought of as inhabiting water, but there are also airborne versions, the aeroplankton, that live part of their lives drifting in the atmosphere. These include plant spores, pollen and wind-scattered seeds, as well as microorganisms swept into the air from terrestrial dust storms and oceanic plankton swept into the air by sea spray. Though many plankton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferricyanide
Ferricyanide is the anion e(CN)6sup>3−. It is also called hexacyanoferrate(III) and in rare, but systematic nomenclature, hexacyanidoferrate(III). The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry. Properties e(CN)6sup>3− consists of a Fe3+ center bound in octahedral geometry to six cyanide ligands. The complex has Oh symmetry. The iron is low spin and easily reduced to the related ferrocyanide ion e(CN)6sup>4−, which is a ferrous (Fe2+) derivative. This redox couple is reversible and entails no making or breaking of Fe–C bonds: : e(CN)6sup>3− + e− ⇌ e(CN)6sup>4− This redox couple is a standard in electrochemistry. Compared to main group cyanides like potassium cyanide, ferricyanides are much less toxic because of the strong bond between the cyanide ion (CN− ) and the Fe3+. They do react with mineral acids, however, to release highly toxic hydrogen cyanide gas. Us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein complex that uses light energy to catalyze the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a proton-motive force that is used to generate ATP. PSI is composed of more than 110 cofactors, significantly more than Photosystem II. History This photosystem is known as PSI because it was discovered before Photosystem II, although future experiments showed that Photosystem II is actually the first enzyme of the photosynthetic electron transport chain. Aspects of PSI were discovered in the 1950s, but the significance of these discoverie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem II
Photosystem II (or water-plastoquinone oxidoreductase) is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen. By replenishing lost electrons with electrons from the splitting of water, photosystem II provides the electrons for all of photosynthesis to occur. The hydrogen ions (protons) generated by the oxidation of water help to create a proton gradient that is used by ATP synthase to generate ATP. The energized electrons transferred to plastoquinone are ultimately used to reduce to NADPH or are used in non-cyclic electron flow. DCMU is a chemical often used in laboratory sett ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thylakoid Membranes
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana (singular: granum). Grana are connected by intergranal/stromal thylakoids, which join granum stacks together as a single functional compartment. In thylakoid membranes, chlorophyll pigments are found in packets called quantasomes. Each quantasome contains 230 to 250 chlorophyll molecules. Etymology The word ''Thylakoid'' comes from the Greek word ''thylakos'' or ''θύλακος'', meaning "sac" or "pouch". Thus, ''thylakoid'' means "sac-like" or "pouch-like". Structure Thylakoids are membrane-bound structures embedded in the chloroplast stroma. A stack of thylakoids is called a granum and resembles a stack of coins. Membrane The thylakoid membrane is the site of the ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Light Harvesting Materials
Light harvesting materials harvest solar energy that can then be converted into chemical energy through photochemical processes. Synthetic light harvesting materials are inspired by photosynthetic biological systems such as light harvesting complexes and pigments that are present in plants and some photosynthetic bacteria. The dynamic and efficient antenna complexes that are present in photosynthetic organisms has inspired the design of synthetic light harvesting materials that mimic light harvesting machinery in biological systems. Examples of synthetic light harvesting materials are dendrimers, porphyrin arrays and assemblies, organic gels, biosynthetic and synthetic peptides, organic-inorganic hybrid materials, and semiconductor materials (non-oxides, oxynitrides and oxysulfides ). Synthetic and biosynthetic light harvesting materials have applications in photovoltaics, photocatalysis, and photopolymerization. Photochemical Processes Organic Photovoltaic Cells During photoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode Voltage
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (SHE). It is defined to have a potential of zero volts. It may also be defined as the potential difference between the charged metallic rods and salt solution. The electrode potential has its origin in the potential difference developed at the interface between the electrode and the electrolyte. It is common, for instance, to speak of the electrode potential of the M+/M redox couple. Origin and interpretation Electrode potential appears at the interface between an electrode and electrolyte due to the transfer of charged species across the interface, specific adsorption of ions at the interface, and specific adsorption/orientation of polar molecules, including those of the solvent. In an electrochemical cell, the cathode and the an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |