|
Bioequivalent
Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same. One article defined bioequivalence by stating that, "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent and their bioavailabilities (rate and extent of availability) after administration in the same molar dose are similar to such a degree that their effects, with respect to both efficacy and safety, can be expected to be essentially the same. Pharmaceutical equivalence implies the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards." For The World Health Organization (WHO) "two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent or pharmaceut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
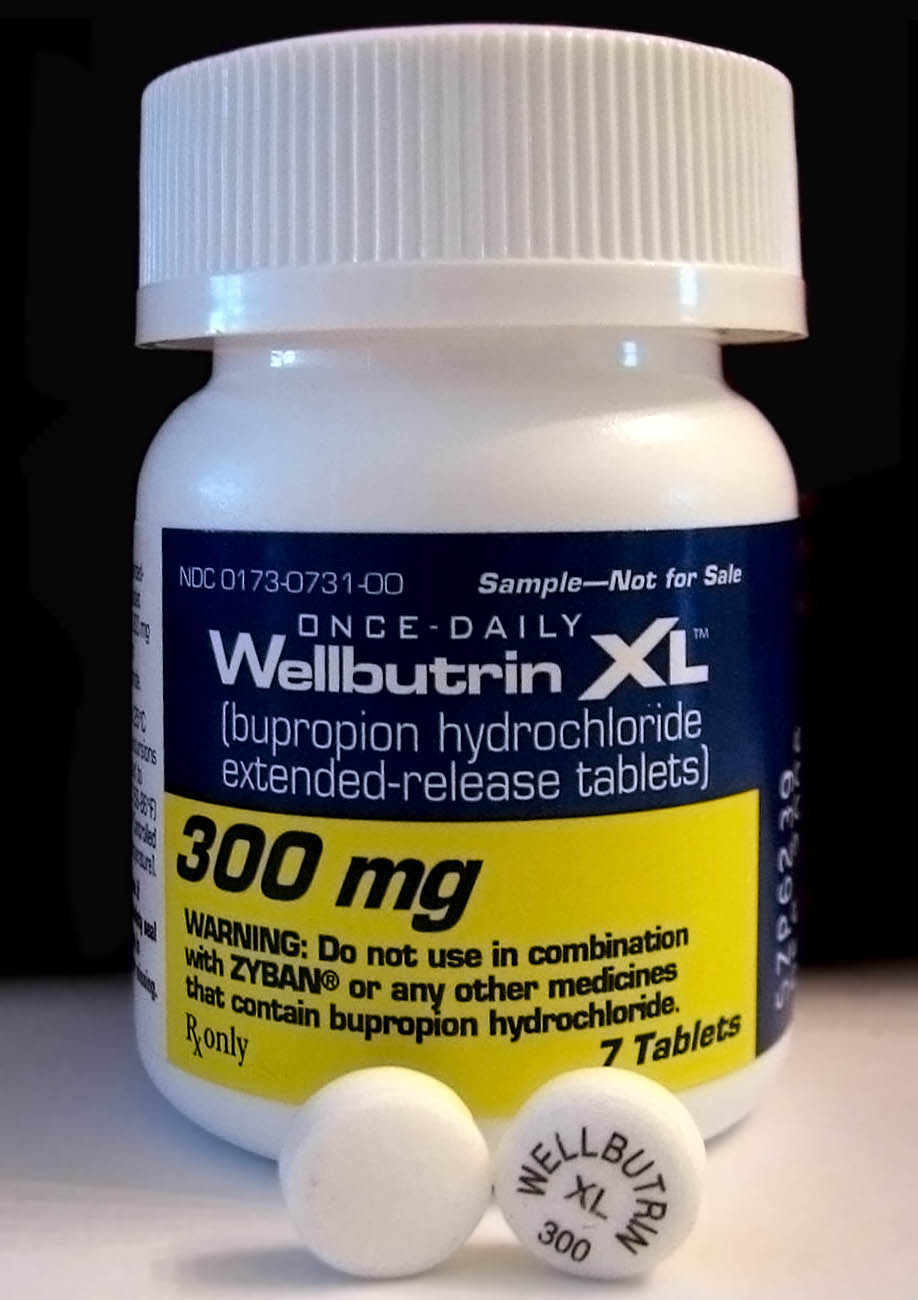
Bupropion Bioequivalency Comparison
Bupropion, formerly called amfebutamone, and sold under the brand name Wellbutrin among others, is an atypical antidepressant that is indicated in the treatment of major depressive disorder, seasonal affective disorder, and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate-release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder. The medication is taken by mouth. Common adverse effects of bupropion with the greatest difference from p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IVIVC
An in-vitro in-vivo correlation (IVIVC) has been defined by the U.S. Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ... (FDA) as "a predictive mathematical model describing the relationship between an in-vitro property of a dosage form and an in-vivo response". Generally, the in-vitro property is the rate or extent of drug dissolution or release while the in-vivo response is the plasma drug concentration or amount of drug absorbed. The United States Pharmacopoeia (USP) also defines IVIVC as "the establishment of a relationship between a biological property, or a parameter derived from a biological property produced from a dosage form, and a physicochemical property of the same dosage form". Typically, the parameter derived from the biological property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levothyroxine
Levothyroxine, also known as -thyroxine, is a synthetic form of the thyroid hormone thyroxine (T4). It is used to treat thyroid hormone deficiency (hypothyroidism), including a severe form known as myxedema coma. It may also be used to treat and prevent certain types of thyroid tumors. It is not indicated for weight loss. Levothyroxine is taken orally (by mouth) or given by intravenous injection. Levothyroxine has a half-life of 7.5 days when taken daily, so about six weeks is required for it to reach a steady level in the blood. Side effects from excessive doses include weight loss, trouble tolerating heat, sweating, anxiety, trouble sleeping, tremor, and fast heart rate. Use is not recommended in people who have had a recent heart attack. Use during pregnancy has been found to be safe. Dosing should be based on regular measurements of thyroid-stimulating hormone (TSH) and T4 levels in the blood. Much of the effect of levothyroxine is following its conversion to tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Warfarin
Warfarin, sold under the brand name Coumadin among others. It is used as an anticoagulant, anticoagulant medication. It is commonly used to prevent deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Warfarin may sometimes be prescribed following a ST-segment elevation myocardial infarction and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously. The common side effect, a natural consequence of reduced clotting, is bleeding. Less common side effects may include areas of tissue necrosis, tissue damage, and purple toes syndrome. Use is not recommended during pregnancy. The effects of warfarin are typically monitored by checking prothrombin time (INR) every one to four weeks. Many other medications and Diet (nutrition), dietary factors can interact with warfarin, either increasing or decreasing its effectiveness. The effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
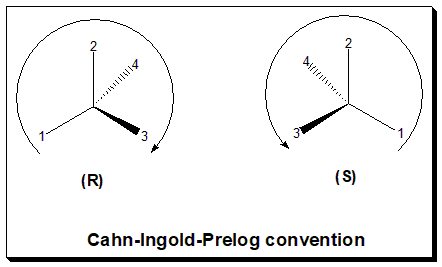
Chiral Drugs
Chemical compounds that come as mirror-image pairs are referred to by chemists as chirality (chemistry), chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called Racemic mixture, racemic drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers chirality (chemistry), chirality to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical composition. Hence "chiral drug" does not say whether the drug is racemic (racemic drug), single enantiomer (chiral specific drug) or some other combination of stereoisomers. To resolve this issue ''Joseph Gal'' introduced a ne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Economic Area
The European Economic Area (EEA) was established via the ''Agreement on the European Economic Area'', an international agreement which enables the extension of the European Union's single market to member states of the European Free Trade Association (EFTA). The EEA links the EU member states and three of the four EFTA states (Iceland, Liechtenstein, and Norway) into an internal market governed by the same EU laws. These rules aim to enable free movement of persons, goods, services, and capital within the European single market, including the freedom to choose residence in any country within this area. The EEA was established on 1 January 1994 upon entry into force of the EEA Agreement. The contracting parties are the EU, its member states, and Iceland, Liechtenstein, and Norway. New members of EFTA would not automatically become party to the EEA Agreement, as each EFTA State decides on its own whether it applies to be party to the EEA Agreement or not. According to Article 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenytoin
Phenytoin (PHT), sold under the brand name Dilantin among others, is an anticonvulsant, anti-seizure medication. It is useful for the prevention of tonic-clonic seizures (also known as grand mal seizures) and focal seizures, but not absence seizures. The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines. It may also be used for certain heart arrhythmias or neuropathic pain. It can be taken intravenously or by mouth. The intravenous form generally begins working within 30 minutes and is effective for roughly 24 hours. Blood levels can be measured to determine the proper dose. Common side effects include nausea, stomach pain, loss of appetite, poor coordination, hypertrichosis, increased hair growth, and Gingival hyperplasia, enlargement of the gums. Potentially serious side effects include sleepiness, self harm, liver problems, bone marrow suppression, hypotension, low blood pressure, toxic epidermal necrolysis, and atrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digoxin
Digoxin (better known as digitalis), sold under the brand name Lanoxin among others, is a medication used to treat various heart disease, heart conditions. Most frequently it is used for atrial fibrillation, atrial flutter, and heart failure. Digoxin is one of the oldest medications used in the field of cardiology. It works by increasing myocardial contractility, increasing stroke volume and blood pressure, reducing heart rate, and somewhat extending the time frame of the Muscle contraction, contraction. Digoxin is taken by mouth or by intravenous, injection into a vein. Digoxin has a half life of approximately 36 hours given at average doses in patients with normal renal function. It is excreted mostly unchanged in the urine. Common side effects include gynecomastia, breast enlargement with other side effects generally due to an excessive dose. These side effects may include loss of appetite, nausea, trouble seeing, confusion, and an Heart arrhythmia, irregular heartbeat. Gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Index
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug with regard to risk of overdose. It is a comparison of the amount of a therapeutic agent that causes toxicity to the amount that causes the therapeutic effect. The related terms therapeutic window or safety window refer to a range of doses optimized between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity. Classically, for clinical indications of an approved drug, TI refers to the ratio of the dose of the drug that causes adverse effects at an incidence/severity not compatible with the targeted indication (e.g. toxic dose in 50% of subjects, TD) to the dose that leads to the desired pharmacological effect (e.g. efficacious dose in 50% of subjects, ED). In contrast, in a drug development setting TI is calculated based on plasma exposure levels. In the early days of pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |