|
Chiral Drugs
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called racemic drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers chirality to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical composition. Hence "chiral drug" does not say whether the drug is racemic (racemic drug), single enantiomer (chiral specific drug) or some other combination of stereoisomers. To resolve this issue ''Joseph Gal'' introduced a new term called ''unichiral.'' Unichiral indicates that the stere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Inversion
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule. Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temperature- induced, etc.) and the energy barrier associated with the stereogenic element present in the chiral molecule. 2-Arylpropionic acid nonsteroidal anti-inflammatory drugs (NSAIDs) provide one of the best pharmaceutical examples of chiral inversion. Chirality is attributed to a molecule due to the presence of a stereogenic element (viz. center, planar, helical, or axis). Many pharmaceutical drugs are chiral and have a labile (configurationally unstable) stereogenic element. Chiral compounds with stereogenic center are found to have high energy barriers for inversion and generally undergo biologically mediated chiral inversion. While compounds with helical or planar chirality have low energy barriers and chiral inversions are often cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethambutol Enantiomers
Ethambutol (EMB, E) is a medication primarily used to treat tuberculosis. It is usually given in combination with other tuberculosis medications, such as isoniazid, rifampicin and pyrazinamide. It may also be used to treat ''Mycobacterium avium'' complex, and ''Mycobacterium kansasii''. It is taken by mouth. Common side effects include problems with vision, joint pain, nausea, headaches, and feeling tired. Other side effects include liver problems and allergic reactions. It is not recommended in people with optic neuritis, significant kidney problems, or under the age of five. Use during pregnancy or breastfeeding has not been found to cause harm. In the United States the FDA has raised concerns about eye issues in the baby if used during pregnancy. Ethambutol is believed to work by interfering with the bacteria's metabolism. Ethambutol was discovered in 1961. It is on the World Health Organization's List of Essential Medicines and is available as a generic medication. Ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopa Enantiomers in Thailand
{{Disambiguation ...
Dopa or DOPA may refer to: * Dihydroxyphenylalanine (DOPA) ** L-DOPA (levodopa), used in the treatment of Parkinson's disease ** D-DOPA, an enantiomer of L-DOPA * 3,4-Dihydroxyphenylacetic acid or DOPAC, a metabolite of dopamine * Dopa, an angel in Enochian * Deleting Online Predators Act of 2006 * Department of Provincial Administration (DOPA), a department under the Ministry of Interior An interior ministry (sometimes called a ministry of internal affairs or ministry of home affairs) is a government department that is responsible for internal affairs. Lists of current ministries of internal affairs Named "ministry" * Ministry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketamine Enantiomers
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a recreational drug. It is one of the safest anesthetics, as, in contrast with opiates, ether, and propofol, it suppresses neither respiration nor heart rate. Ketamine is also simple to administer and highly tolerable compared to drugs with similar effects which are flammable, irritating, or even explosive. Ketamine is a novel compound, derived from PCP, created in pursuit of a safer anesthetic with similar characteristics. Ketamine is also used for acute pain management. At anesthetic doses, ketamine induces a state of "dissociative anesthesia", a trance-like state providing pain relief, sedation, and amnesia. The distinguishing features of ketamine anesthesia are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation. At lower, sub-anesthetic doses, ketamine is a promising agent for p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillamine
Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, and various heavy metal poisonings. It is taken by mouth. Penicillamine was approved for medical use in the United States in 1970. It is on the World Health Organization's List of Essential Medicines. Medical uses It is used as a chelating agent: * In Wilson's disease, a rare genetic disorder of copper metabolism, penicillamine treatment relies on its binding to accumulated copper and elimination through urine. * Penicillamine was the second line treatment for arsenic poisoning, after dimercaprol (BAL). It is no longer recommended. In cystinuria, a hereditary disorder in which high urine cystine levels lead to the formation of cystine stones, penicillamine binds with cysteine to yield a mixed disulfide which is more soluble than cys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillamine Enantiomers
Penicillamine, sold under the brand name of Cuprimine among others, is a medication primarily used for the treatment of Wilson's disease. It is also used for people with kidney stones who have high urine cystine levels, rheumatoid arthritis, and various heavy metal poisonings. It is taken by mouth. Penicillamine was approved for medical use in the United States in 1970. It is on the World Health Organization's List of Essential Medicines. Medical uses It is used as a chelating agent: * In Wilson's disease, a rare genetic disorder of copper metabolism, penicillamine treatment relies on its binding to accumulated copper and elimination through urine. * Penicillamine was the second line treatment for arsenic poisoning, after dimercaprol (BAL). It is no longer recommended. In cystinuria, a hereditary disorder in which high urine cystine levels lead to the formation of cystine stones, penicillamine binds with cysteine to yield a mixed disulfide which is more soluble than cystine. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
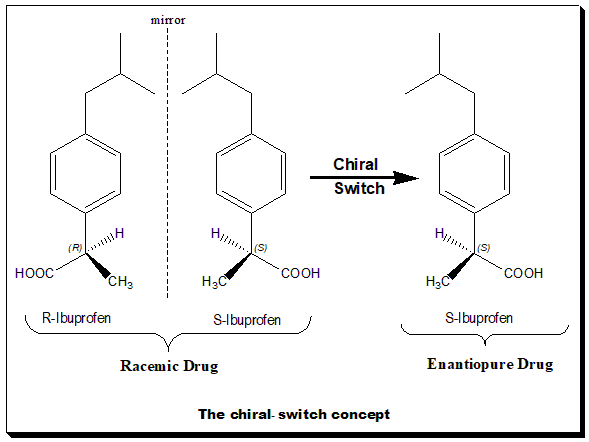
Chiral Switch
The word "chiral switch" was introduced by Agranat and Caner in 1999. Chiral switches are chiral drugs that are already approved as racemates but that have been re-developed as single enantiomers. The term chiral switching has been coined to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Inversion
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule. Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temperature- induced, etc.) and the energy barrier associated with the stereogenic element present in the chiral molecule. 2-Arylpropionic acid nonsteroidal anti-inflammatory drugs (NSAIDs) provide one of the best pharmaceutical examples of chiral inversion. Chirality is attributed to a molecule due to the presence of a stereogenic element (viz. center, planar, helical, or axis). Many pharmaceutical drugs are chiral and have a labile (configurationally unstable) stereogenic element. Chiral compounds with stereogenic center are found to have high energy barriers for inversion and generally undergo biologically mediated chiral inversion. While compounds with helical or planar chirality have low energy barriers and chiral inversions are often cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibuprofen
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used for treating pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be used by mouth or intravenously. It typically begins working within an hour. Common side effects include heartburn and a rash. Compared to other NSAIDs, it may have other side effects such as gastrointestinal bleeding. It increases the risk of heart failure, kidney failure, and liver failure. At low doses, it does not appear to increase the risk of heart attack; however, at higher doses it may. Ibuprofen can also worsen asthma. While whether it is safe in early pregnancy is unclear, it appears to be harmful in later pregnancy, so is not recommended. Like other NSAIDs, it works by inhibiting the production of prostaglandins by decreasing the activity of the enzyme cyclooxygenase (COX). Ibuprofen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propoxyphene
Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive (cough suppressant) and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia (pain relief) is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration. Dextropropoxyphene is sometimes combined with acetaminophen. Trade names include Darvocet-N, Di-Gesic, and Darvon with APAP (for dextropropoxyphene and paracetamol). The British approved name (i.e. the generic name of the active ingredient) of the paracetamol/dextropropoxyphene preparation is co-proxamol (sold under a vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indacrinone
Indacrinone is a loop diuretic. It can be used in patients of gout with hypertension as an antihypertensive because it decreases reabsorption of uric acid, while other diuretics increase it. Chirality and biological activity Indacrinone is a chiral drug, with one chiral center and hence exists as mirror-image twins. (R)-enantiomer, the eutomer, is diuretic whereas the mirror-image version (S)-enantiomer counteracts side effect of the eutomer. Here both the enantiomers contribute to the overall desired effect in different ways. As indicated earlier, the (R)- enantiomer is the pharmacologically active diuretic. Like most other diuretics, the (R)-isomer possesses an undesirable side-effect of retaining uric acid. But the (S)-enantiomer, the distomer, has the property of assisting uric acid secretion (uricosuric effect), and, therefore, antagonizing the undesirable side-effects of the eutomer (uric-acid retention). It affords a good argument for the marketing of a racemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |