|
Binding Protein
A binding protein is any protein that acts as an agent to bind two or more molecules together. Examples include: *DNA-binding protein **Single-strand binding protein **Telomere-binding protein *RNA-binding protein **Poly(A)-binding protein ** Nuclear cap-binding protein complex * CREB-binding protein *Calcium-binding protein **Calcium-binding protein 1 ** S100 calcium-binding protein A1 *TATA-binding protein * Actin-binding protein Most actin binding proteins bind on the actin surface, despite having different functions and structures. *Penicillin binding proteins *Retinol binding protein **Retinol binding protein 4 * EP300 *Binding immunoglobulin protein *Odorant binding protein *Lipopolysaccharide-binding protein *C4b-binding protein *Rap GTP-binding protein *Calmodulin-binding proteins *Iron-binding proteins *Thyroxine-binding proteins *Folate-binding protein *Sterol regulatory element-binding protein *GTP-binding protein *Retinaldehyde-binding protein 1 *Ccaat-enhancer-bind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
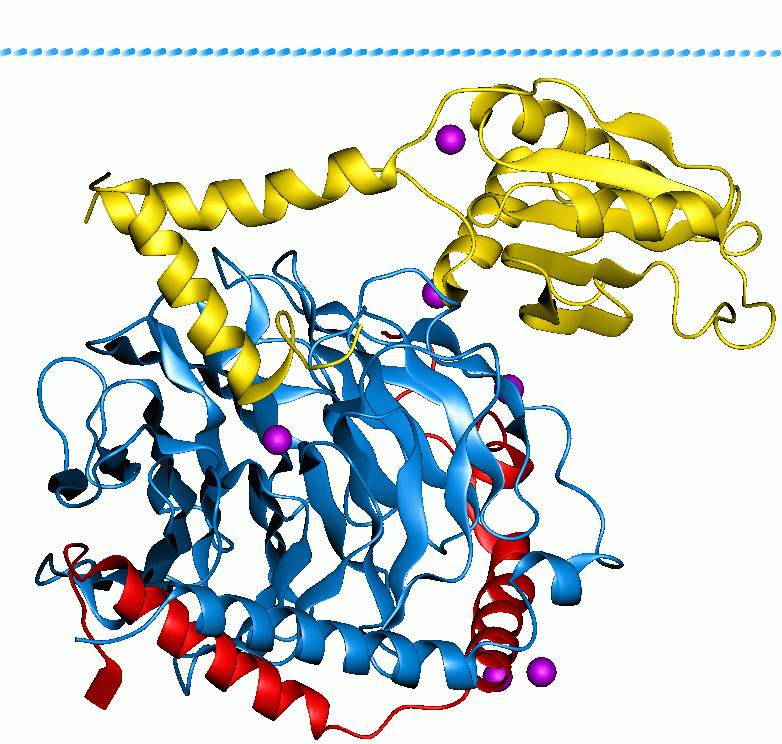
EP300
Histone acetyltransferase p300 also known as p300 HAT or E1A-associated protein p300 (where E1A = adenovirus early region 1A) also known as EP300 or p300 is an enzyme that, in humans, is encoded by the ''EP300'' gene. It functions as histone acetyltransferase that regulates transcription of genes via chromatin remodeling by allowing histone proteins to wrap DNA less tightly. This enzyme plays an essential role in regulating cell growth and division, prompting cells to mature and assume specialized functions (differentiate), and preventing the growth of cancerous tumors. The p300 protein appears to be critical for normal development before and after birth. The EP300 gene is located on the long (q) arm of the human chromosome 22 at position 13.2. This gene encodes the adenovirus E1A-associated cellular p300 transcriptional co-activator protein. EP300 is closely related to another gene, CREB binding protein, which is found on human chromosome 16. Function p300 HAT functions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ccaat-enhancer-binding Proteins
CCAAT-enhancer-binding proteins (or C/EBPs) is a family of transcription factors composed of six members, named from C/EBPα to C/EBPζ. They promote the expression of certain genes through interaction with their promoters. Once bound to DNA, C/EBPs can recruit so-called co-activators (such as CBP) that in turn can open up chromatin structure or recruit basal transcription factors. Function C/EBP proteins interact with the CCAAT (cytosine-cytosine- adenosine-adenosine-thymidine) box motif, which is present in several gene promoters. They are characterized by a highly conserved basic-leucine zipper (bZIP) domain at the C-terminus. This domain is involved in dimerization and DNA binding, as are other transcription factors of the leucine zipper domain-containing family ('' c-Fos'' and '' c-jun''). The bZIP domain structure of C/EBPs is composed of an α-helix that forms a "coiled coil" structure when it dimerizes. Members of the C/EBP family can form homodimers or heterodime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinaldehyde-binding Protein 1
Retinaldehyde-binding protein 1 (RLBP1) also known as cellular retinaldehyde-binding protein (CRALBP) is a 36-kD water-soluble protein that in humans is encoded by the ''RLBP1'' gene. Discovery Cellular retinol binding protein (CRBP) was first discovered in 1973 from lung tissues by Bashor et al. There have been three cellular retinol binding protein categories discovered; Cellular retinol-binding protein, cellular retinoic acid-binding protein and cellular retinaldehyde-binding protein(CRALBP). CRALBP was first discovered in 1977, after it was purified from retina and retinal pigment epithelial cells. Function The cellular retinaldehyde-binding protein transports 11-cis-retinal (also known as 11-cis-retinaldehyde) as its physiological ligands. It plays a critical role as an 11-cis-retinal acceptor which facilitates the enzymatic isomerization of all 11-trans-retinal to 11-cis-retinal, in the isomerization of the rod and cones of the visual cycle. Tissue distribution CRAL ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTP-binding Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol Regulatory Element-binding Protein
Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes ''SREBF1'' and ''SREBF2''. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors. Unactivated SREBPs are attached to the nuclear envelope and endoplasmic reticulum membranes. In cells with low levels of sterols, SREBPs are cleaved to a water-soluble N-terminal domain that is translocated to the nucleus. These activated SREBPs then bind to specific sterol regulatory element DNA sequences, thus upregulating the synthesis of enzymes involved in sterol biosynthesis. Sterols in turn inhibit the cleavage of SREBPs and therefore synthesis of additional sterols is reduced through a negative feed back loop. Isoforms Mammalian genomes have two separate SREBP genes ( and ): * SREBP-1 expression produces two different isoforms, SREBP-1a and -1c. These isoforms differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Folate-binding Protein
Folate-binding protein (FBP) are proteins that bind folate, typically the folate receptors and reduced folate carrier. FBP can be a marker for ovarian cancer. FBP can be measured to help in tumour classification to aid treatment, as it is over-expressed (20-80 fold) in more than 90% of ovarian and endometrial cancers, as well as 20-50% of breast, lung, colorectal, and renal cell carcinomas. FBP has very limited tissue distribution and expression in non-malignant tissue, making it a good immunotherapy target.http://www.marketwatch.com/story/galena-biopharma-to-present-at-the-10th-annual-biotechnology-investor-forum-2011-10-21 Alternative usage FBP can refer to protein(s) (e.g. extracted from cow's milk) used to do folic acid Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and ... assay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyroxine-binding Proteins
A thyroxine-binding protein is any of several transport proteins that bind thyroid hormone and carry it around the bloodstream. Examples include: * Thyroxine-binding globulin * Transthyretin * Serum albumin Serum albumin, often referred to simply as blood albumin, is an albumin (a type of globular protein) found in vertebrate blood. Human serum albumin is encoded by the ''ALB'' gene. Other mammalian forms, such as bovine serum albumin, are chemical ... External links * Human proteins Blood proteins {{Protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-binding Proteins
Iron-binding proteins are carrier proteins and metalloproteins that are important in iron metabolism and the immune response. Iron is required for life. Iron-dependent enzymes catalyze a variety of biochemical reactions and can be divided into three broad classes depending on the structure of their active site: non-heme mono-iron, non-heme diiron , or heme centers. A well-known family of iron-dependent enzymes include oxygenases that facilitate hydroxyl group addition of one or both atoms from o2. Notable enzymes include tryptophan dioxygenase, ferredoxin, and 2-oxoglutarate dioxygenase. Heme proteins Heme proteins are proteins that contain a heme prosthetic group. The heme group consists of a porphyrin ring coordinated with an iron ion. Four nitrogen atoms in the porphyrin ring act as a ligand for the iron in the center. In many cases, the equatorial porphyrin is complemented by one or two axial ligands. An example of this is in hemoglobin, where the porphyrin works together wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin-binding Proteins
Calmodulin-binding proteins are, as their name implies, proteins which bind calmodulin. Calmodulin can bind to a variety of proteins through a two-step binding mechanism, namely "conformational and mutually induced fit", where typically two domains of calmodulin wrap around an emerging helical calmodulin binding domain from the target protein. Examples include: * Gap-43 protein (presynaptic) * Neurogranin (postsynaptic) * Caldesmon Ca2+ Activation A variety of different ions, including Calcium (Ca2+), play a vital role in the regulation of cellular functions. Calmodulin, a Calcium-binding protein, that mediates Ca2+ signaling is involved in all types of cellular mechanisms, including metabolism, synaptic plasticity, nerve growth, smooth muscle contraction, etc. Calmodulin allows for a number of proteins to aid in the progression of these pathways using their interactions with CaM in its Ca2+-free or Ca2+-bound state. Proteins each have their own unique affinities for calmodul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rap GTP-binding Protein
Rap GTP-binding protein also known as Ras-related proteins or simply RAP is a type of small GTPase, similar in structure to Ras. These proteins share approximately 50% amino acid identity with the classical RAS proteins and have numerous structural features in common. The most striking difference between RAP proteins and RAS proteins resides in their 61st amino acid: glutamine in RAS is replaced by threonine in RAP proteins. RAP counteracts the mitogenic function of RAS because it can interact with RAS GAPs and RAF in a competitive manner. Family members Human genes that encode Ras-related proteins include: * RAP1A, RAP1B * RAP2A, RAP2B, RAP1C * RAB5C Ras-related protein Rab-5C is a protein that in humans is encoded by the ''RAB5C'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' o ... References External links * EC 3.6.5 Peripheral membrane prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C4b-binding Protein
C4b-binding protein (C4BP) is a protein complex involved in the complement system where it acts as inhibitor. C4BP has an octopus-like structure with a central stalk and seven branching alpha-chains. The main form of C4BP in human blood is composed of 7 identical alpha-chains and one unique beta-chain, which in turn binds anticoagulant, vitamin K-dependent protein S. C4BP is a large glycoprotein (500 kDa) with an estimated plasma concentration of 200 micrograms/mL synthesized mainly in the liver. The genes coding for C4BP α-chain (C4BPA) and β-chain (C4BPB) are located in the regulators of complement activation (RCA) gene cluster on the long arm of chromosome 1 in the vicinity of other complement inhibitors. Functions It inhibits the action the classical and the lectin pathways, more specifically C4. It also has ability to bind C3b. C4BP accelerates decay of C3-convertase and is a cofactor for serine protease factor I which cleaves C4b and C3b. C4BP binds apoptotic and n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |