|
Bicarbonate Transporter Protein
The anion exchanger familyTC# 2.A.31 also named bicarbonate transporter family) is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria (e.g., in ''Enterococcus faecium'', 372 aas; gi 22992757; 29% identity in 90 residues). Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in thTransporter Classification Database. Family overview Bicarbonate (HCO3 −) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stilbenes
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5- ''E'')-2-phenylvinylenzene-1,3-diol, biosynthesized by the Gram-negative bacterium '' Photorhabdus luminescens.'' Chemistry Stilbenoids are hydroxylated derivatives of stilbene and have a C6–C2–C6 structure. They belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Under UV irradiation, stilbene and its derivatives undergo intramolecular cyclization, called stilbene photocyclization to form dihydrophenanthrenes. Oligomeric forms are known as oligostilbenoids. Types ;Aglycones * Piceatannol in the roots of Norway spruces * Pinosylvin is a fungal toxin protecting woo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldolase
Fructose-bisphosphate aldolase (), often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism. The word aldolase also refers, more generally, to an enzyme that performs an aldol reaction (creating an aldol) or its reverse (cleaving an aldol), such as Sialic acid aldolase, which forms sialic acid. See the list of aldolases. Mechanism and structure Class I proteins form a protonated Schiff base intermediate linking a highly conserved active site lysine with the DHAP carbonyl carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis. Function The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. Phosphofructokinase catalyses the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate, a key regulatory step in the glycolytic pathway. It is allosterically inhibited by ATP and allosterically activated by AMP, thus indicating the cell's energetic needs when it undergoes the glycolytic pathway. PFK exists as a homotetramer in bacteria and mammals (where each monomer possesses 2 similar domains) and as an octomer in yeast (where there are 4 alpha- (PFK1) and 4 beta-chains (PFK2), the latter, like the mammalian monomers, possessing 2 similar domains). This protein may use the morpheein model of allosteric regulation. PFK is about 300 amino acids in length, and structural studies of the bacterial enzyme have shown it comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyceraldehyde-3-phosphate Dehydrogenase
Glyceraldehyde 3-phosphate dehydrogenase (abbreviated GAPDH) () is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including transcription activation, initiation of apoptosis, ER to Golgi vesicle shuttling, and fast axonal, or axoplasmic transport. In sperm, a testis-specific isoenzyme GAPDHS is expressed. Structure Under normal cellular conditions, cytoplasmic GAPDH exists primarily as a tetramer. This form is composed of four identical 37-kDa subunits containing a single catalytic thiol group each and critical to the enzyme's catalytic function. Nuclear GAPDH has increased isoelectric point (pI) of pH 8.3–8.7. Of note, the cysteine residue C152 in the enzyme's active site is required for the induction of apoptosis by oxidative stress. Notably, pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
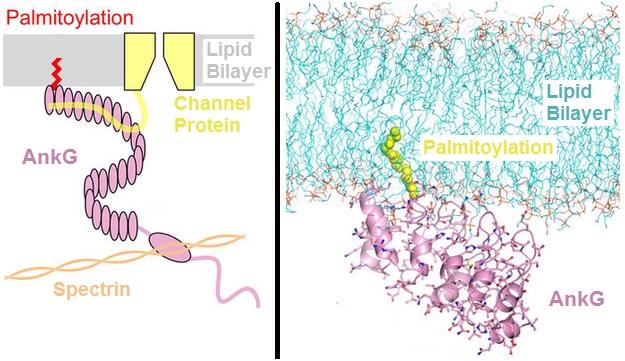
Ankyrin
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Blood Cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "hollow vessel", with ''-cyte'' translated as "cell" in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries. The cytoplasm of a red blood cell is rich in hemoglobin, an iron-containing biomolecule that can bind oxygen and is responsible for the red color of the cells and the blood. Each human red blood cell contains approximately 270 million hemoglobin molecules. The cell membrane is composed of proteins and lipids, and this structure provides properties essential for phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band 3
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the gene in humans. Band 3 anion transport protein is a phylogenetically-preserved transport protein responsible for mediating the exchange of chloride (Cl−) with bicarbonate (HCO3−) across plasma membranes. Functionally similar members of the AE clade are AE2 and AE3. Function Band 3 is present in the basolateral face of the α-intercalated cells of the collecting ducts of the nephron, which are the main acid-secreting cells of the kidney. They generate hydrogen ions and bicarbonate ions from carbon dioxide and water – a reaction catalysed by carbonic anhydrase. The hydrogen ions are pumped into the collecting duct tubule by vacuolar H+ ATPase, the apical proton pump, which thus excretes acid into the urine. kAE1 exchanges bicarbonate for chloride on the basolateral surface, essentially returning bicarbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Sequence
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant
Plants are predominantly Photosynthesis, photosynthetic eukaryotes of the Kingdom (biology), kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclude the fungi and some algae, as well as the prokaryotes (the archaea and bacteria). By one definition, plants form the clade Viridiplantae (Latin name for "green plants") which is sister of the Glaucophyte, Glaucophyta, and consists of the green algae and Embryophyte, Embryophyta (land plants). The latter includes the flowering plants, conifers and other gymnosperms, ferns and Fern ally, their allies, hornworts, liverworts, and mosses. Most plants are multicellular organisms. Green plants obtain most of their energy from sunlight via photosynthesis by primary chloroplasts that are derived from endosymbiosis with cyanobacteria. Their chloroplasts contain chlorophylls a and b, which gives them their green colo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |