|
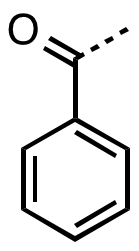
Benzoyl Fluoride
In organic chemistry, benzoyl (, ) is the functional group with the formula C6H5CO-. It can be viewed as benzaldehyde missing one hydrogen. The term "benzoyl" should not be confused with benzyl, which has the formula C6H5CH2. The benzoyl group is given the symbol "Bz". Benzyl is commonly abbreviated "Bn". Sources Benzoyl chloride is a favored source of benzoyl groups, being used to prepare benzoyl ketones, benzamides (benzoyl amides), and benzoate esters. The source of many naturally occurring benzoyl compounds is the thioester benzoyl-CoA. Irradiation of benzil generates benzoyl radicals, which have the formula PhCO. Benzoyl compounds Many ketones contain the benzoyl group. They have the formula C6H5CO–R, an important example being benzophenone. Benzoyl esters and amides are common in organic chemistry. The esters are used as a protecting groups in organic synthesis, which can be easily removed by hydrolysis in dilute basic BASIC (Beginners' All-purpose Symbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important plastics l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radikal Benzoil
''Radikal'' () was a daily liberal Turkish language newspaper, published in Istanbul. From 1996 it was published by Aydın Doğan's Doğan Media Group. Although Radikal did not endorse a particular political alignment, it was generally considered by the public as a social liberal newspaper. Despite only having a circulation of around 25,000 (July 2013), it was considered one of the most influential Turkish newspapers. It was praised for its culture, arts, and interview sections, as well as columnists such as M. Serdar Kuzuloğlu, Hakkı Devrim, Yıldırım Türker, Türker Alkan, Tarhan Erdem, Cengiz Çandar, and Altan Öymen. Hasan Celal Güzel, former minister of national education, Murat Yetkin, and Mustafa Akyol, son of Taha Akyol, also write for Radikal. On 22 March 2016, it was announced that the newspaper was shutting down by the end of the month due to financial reasons. History Radikal was founded in 1996, and "within a decade ... had become one of the most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pteris Ensiformis
''Pteris ensiformis'', the slender brake, silver lace fern, sword brake fern, or slender brake fern, is a plant species of the genus Pteris in the family Pteridaceae. It is found in Asia and the Pacific. Uses Beverages It is the most common ingredient of traditional herbal drinks in Taiwan containing different phenolic compounds : kaempferol 3-O-α-l-rhamnopyranoside-7-O- �-d-apiofuranosyl-(1-2)-β-d-glucopyranoside 7-O- caffeoyl hydroxymaltol 3-O-β-d-glucopyranoside, hispidin 4-O-β-d-glucopyranoside, kaempferol 3-O-α-l-rhamnopyranoside-7-O-β-d-glucopyranoside, caffeic acid, 5-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid. This plant is resistant to arsenic-induced oxidative stress. Benzoyl-beta-D-glucoside, as well as pterosin sesquiterpenes can be found in ''P. ensiformis''. Cultivation ''Pteris ensiformis'' is cultivated as an ornamental plant for tropical and subtropical climate gardens, and as a house plant. ;Cultivars * ''Pt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoyl-beta-D-glucoside
Benzoyl-''beta''--glucoside is a benzoyl glucoside, a natural substance that can be found in ''Pteris ensiformis ''Pteris ensiformis'', the slender brake, silver lace fern, sword brake fern, or slender brake fern, is a plant species of the genus Pteris in the family Pteridaceae. It is found in Asia and the Pacific. Uses Beverages It is the most common ing ...''. References Benzoate esters Glucosides {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basic (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone. Uses Benzophenone can be used as a photo initiator in UV(Ultra-violet)-curing applications such as inks, imaging, and clear coatings in the printing industry. Benzophenone prevents ultraviolet ( UV) light from damaging scents and colors in products such as perfumes and soaps. Benzophenone can also be added to plastic packaging as a UV blocker to prevent photo-degradation of the packaging polymers or its contents. Its use allows manufacturers to package the product in clear glass or plastic (such as a PETE water bottle). Without it, opaque or dark packaging would be required. In biological applications, benzophenones have been used extensively as photophysical probes to identify and map peptide–protein interactions. Benzophenone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |