|
Benzocyclobutadiene
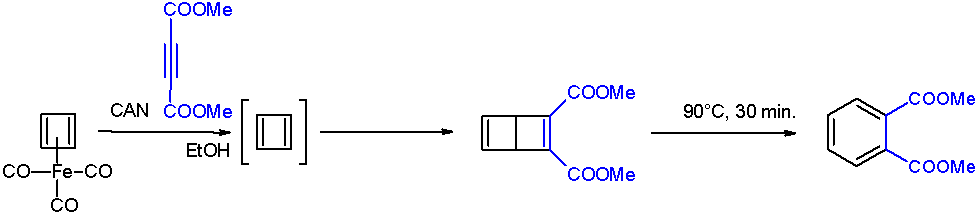
Benzocyclobutadiene is the simplest polycyclic hydrocarbon, being composed of an aromatic benzene ring fused to an anti-aromatic cyclobutadiene ring. It has chemical formula . Though the benzene ring is stabilized by aromaticity, the cyclobutadiene portion has a destabilizing effect. This results into it being a non-aromatic compound - neither behaving as aromatic nor an antiaromatic one. For this reason, benzocyclobutadiene will readily dimerize or polymerize and it reacts as a dienophile in Diels-Alder reactions. Benzocyclobutadiene is used in the production of the pharmaceutical drug naflocort. See also *Pentalene Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula . It is antiaromatic, because it has 4''n'' π electrons where ''n'' is any integer. For this reason it dimerizes even at temperatures as ... References {{reflist Polycyclic aromatic hydrocarbons Bicyclic compounds Four-membered rings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentalene
Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula . It is antiaromatic, because it has 4''n'' π electrons where ''n'' is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-''tert''-butylpentalene was synthesized in 1973. Because of the ''tert''-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene. Dilithium pentalenide was isolated in 1962, long before pentalene itself in 1997. It is prepared from reaction of dihydropentalene (pyrolysis of an isomer of dicyclopentadiene) with ''n''-butyllithium in solution and forms a stable salt. In accordance with its structure proton NMR shows 2 signals in a 2 to 1 ratio. The addition of two electrons removes the antiaromaticity; it becomes a planar 10π-electron aromatic species and is thus a bicyclic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept. By definition, polycyclic aromatic hydrocarbons have multiple rings, precluding benzene from being considered a PAH. Some sources, such as the US EPA and CDC, consider naphthalene to be the simplest PAH. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many non-be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PVC), whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naflocort
Naflocort (developmental code name SQ-26490) is a synthetic glucocorticoid corticosteroid Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involv ... which was never marketed. References Diketones Diols Organofluorides Glucocorticoids Tetralins Pregnanes Abandoned drugs {{steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Aromatic Hydrocarbons
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept. By definition, polycyclic aromatic hydrocarbons have multiple rings, precluding benzene from being considered a PAH. Some sources, such as the US EPA and CDC, consider naphthalene to be the simplest PAH. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)
.png)
