|
Benserazide
Benserazide (also called Serazide or Ro 4-4602) is a peripherally acting aromatic L-amino acid decarboxylase or DOPA decarboxylase inhibitor, which is unable to cross the blood–brain barrier. It is on the World Health Organization's List of Essential Medicines. Indications It is used in the management of Parkinson's disease in combination with L-DOPA (levodopa) as co-beneldopa ( BAN), under the brand names Madopar in the UK and Prolopa in Canada, both made by Roche. Benserazide is not approved for use in the US; carbidopa is used, instead, for the same purpose. These combinations are also used for the treatment of restless leg syndrome.Ryan, Melody; Slevin, John T. (2006)"Restless legs syndrome" ''American Journal of Health-System Pharmacy''. 63 (17): 1599-1612. Retrieved on 2008-02-06. Pharmacology Levodopa is a precursor to the neurotransmitter dopamine, which is administered to increase its levels in the central nervous system. However, most levodopa is decarboxyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOPA Decarboxylase Inhibitor
An aromatic L-amino acid decarboxylase inhibitor (synonyms: DOPA decarboxylase inhibitor, Extracerebral decarboxylase inhibitor, DDCI and AAADI) is a medication of type enzyme inhibitor which inhibits the synthesis of dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC, AAAD, or DOPA decarboxylase). It is used to inhibit the decarboxylation of L-DOPA to dopamine outside the brain, i.e. in the blood. This is primarily co-administered with L-DOPA to combat Parkinson's disease. Administration can prevent common side-effects, such as nausea and vomiting, as a result of interaction with D2 receptors in the vomiting center (or cheomoreceptor trigger zone) located outside the blood–brain barrier. Examples of extracerebral decarboxylase inhibitors include carbidopa and benserazide. Indications Peripherally selective DDCIs incapable of crossing the protective blood–brain barrier (BBB) are used in augmentation of L-DOPA (levodopa) in the treatment of Parkinson' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levodopa
-DOPA, also known as levodopa and -3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of some plants and animals, including humans. Humans, as well as a portion of the other animals that utilize -DOPA, make it via biosynthesis from the amino acid -tyrosine. -DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, -DOPA itself mediates neurotrophic factor release by the brain and CNS. -DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, and Stalevo. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia. -DOPA has a counterpart with opposite chirality, -DOPA. As is true for many molecules, the human body produces only one of these isomers (the -DOPA form). The enant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vasoconstriction
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessels. The process is particularly important in controlling hemorrhage and reducing acute blood loss. When blood vessels constrict, the flow of blood is restricted or decreased, thus retaining body heat or increasing vascular resistance. This makes the skin turn paler because less blood reaches the surface, reducing the radiation of heat. On a larger level, vasoconstriction is one mechanism by which the body regulates and maintains mean arterial pressure. Medications causing vasoconstriction, also known as vasoconstrictors, are one type of medicine used to raise blood pressure. Generalized vasoconstriction usually results in an increase in systemic blood pressure, but it may also occur in specific tissues, causing a localized reduction in bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with . They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and prevent reuptak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the targe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation. Phenethylamine is sold as a dietary supplement for purported mood and weight loss-related therapeutic benefits; however, in orally ingested phenethylamine, a significant amount is metabolized in the small intestine by monoami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its analgesic and antidepressant effects. It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement. As an essential amino acid, phenylalanine is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
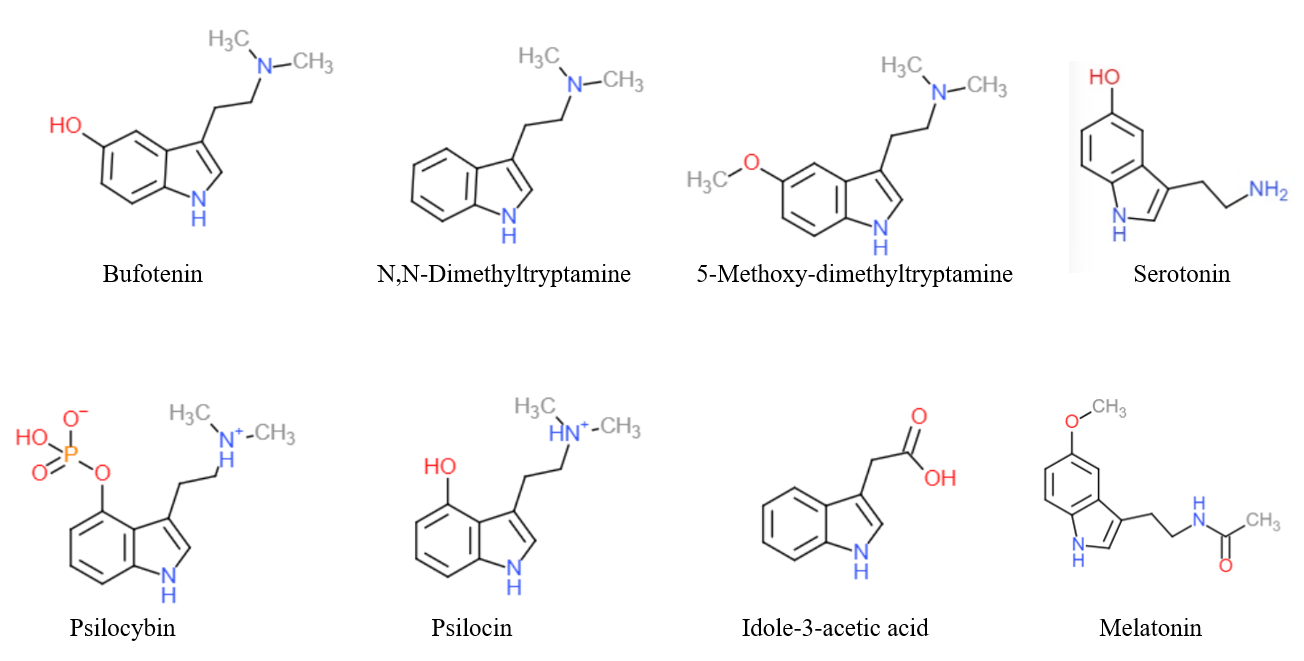
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
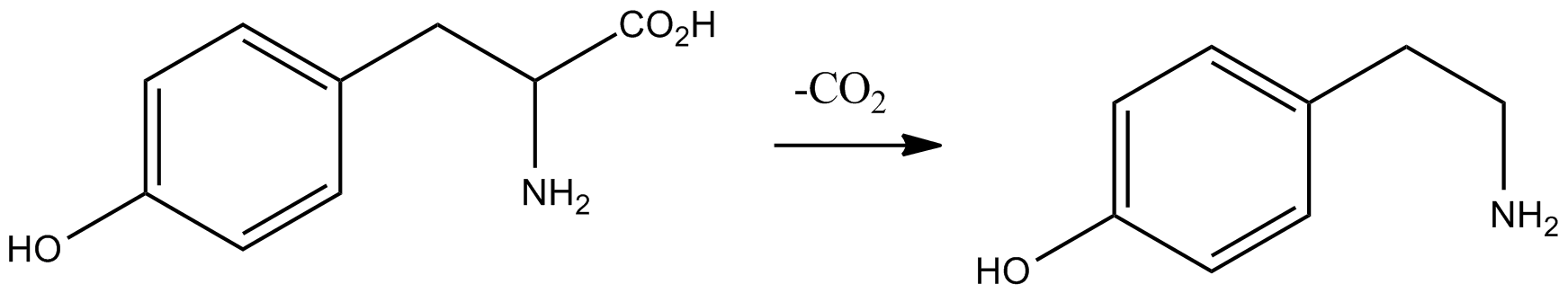
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HTP
5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin. Uses 5-HTP is sold over the counter in the United States, Canada, Singapore, the Netherlands, and the United Kingdom as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. It is also marketed in many European countries for the indication of major depression under the trade names Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum. A 2002 review concluded that although the data evaluated suggests that 5-HTP is more effective than placebo in the treatment of depression, the evidence was insufficient to be conclusive due to a lack of clinical data meeting the rigorous standards of the day. More and larger studies using current methodologies are needed to determine if 5-HTP is truly effective in treating depression. In small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |