|
Bay R 1531
Bay R 1531 is a tricyclic tryptamine derivative which acts as a selective serotonin receptor 5-HT1A agonist. It was researched unsuccessfully for the treatment of stroke but remains in use for scientific research. See also * 8-OH-DPAT * RDS-127 * RU-28306 * LY-293,284 LY-293284 is a research chemical developed by the pharmaceutical company Eli Lilly and used for scientific studies. It acts as a potent and selective 5-HT1A receptor full agonist. It was derived through structural simplification of the ergoline ... * NDTDI References {{Tryptamines Serotonin receptor agonists Tryptamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
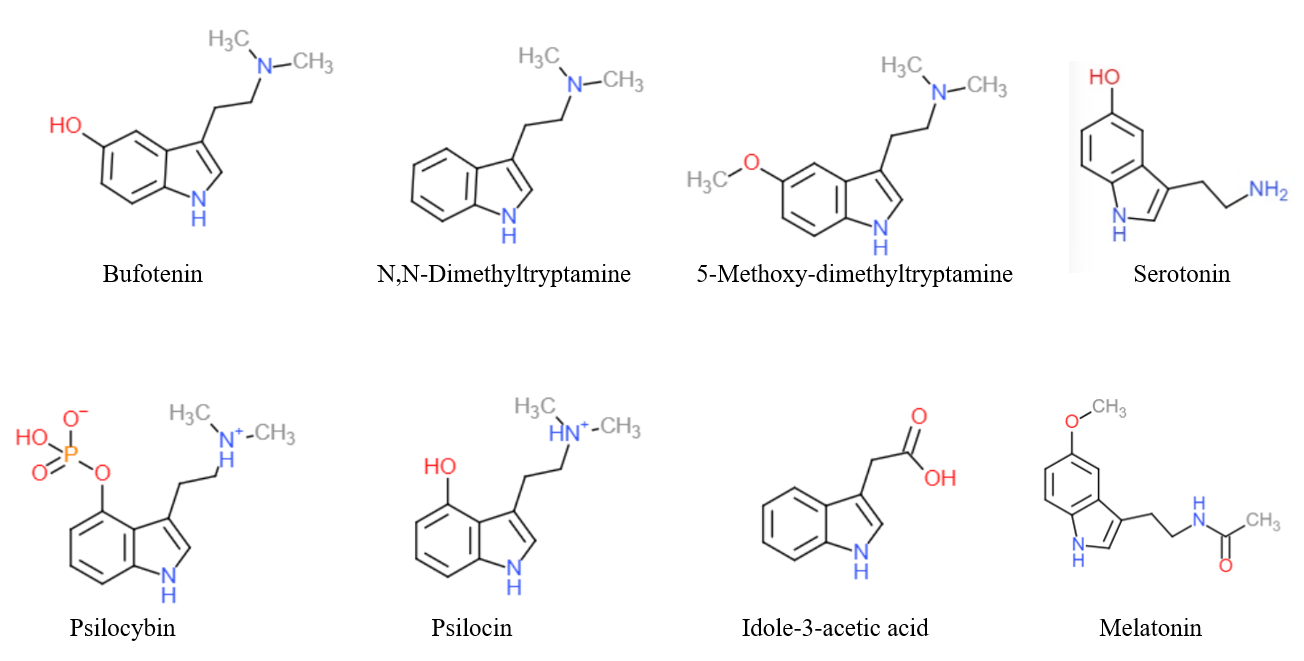
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. They are the target of a variety of pharmaceutical and recreational drugs, including many antidepressants, antipsychotics, anorectics, antiemetics, gast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1A Receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, septum, amygdala, and raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neuromodulation 5-HT1A recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly. Signs and symptoms of a stroke may include an inability to move or feel on one side of the body, problems understanding or speaking, dizziness, or loss of vision to one side. Signs and symptoms often appear soon after the stroke has occurred. If symptoms last less than one or two hours, the stroke is a transient ischemic attack (TIA), also called a mini-stroke. A hemorrhagic stroke may also be associated with a severe headache. The symptoms of a stroke can be permanent. Long-term complications may include pneumonia and loss of bladder control. The main risk factor for stroke is high blood pressure. Other risk factors include high blood cholesterol, tobacco smoking, obesity, diabetes mellitus, a previous TIA, end-st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
8-OH-DPAT
8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered. Originally believed to be selective for the 5-HT1A receptor, 8-OH-DPAT was later found to act as a 5-HT7 receptor agonist and serotonin reuptake inhibitor/releasing agent as well. In animal studies, 8-OH-DPAT has been shown to possess antidepressant, anxiolytic, serenic, anorectic, antiemetic, hypothermic, hypotensive, bradycardic, hyperventilative, and analgesic effects. See also * 5-OH-DPAT * 7-OH-DPAT * Bay R 1531 * MDAT * UH-301 (S)-UH-301 is a drug and research chemical widely used in scientific studies. It acts as a selective 5-HT1A receptor silent antagonist. It is structurally related to 8-OH-DPAT. UH-301 was found to produce a head-twitch response in mice which is ... References External links * Yves ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RDS-127
RDS-127 is a drug which is used in scientific research. It acts as a D2-like receptor agonist and also has some serotonin and adrenergic agonist effects, as well as some anticholinergic action, and produces both anorectic and pro-sexual effects in animal studies. See also * Bay R 1531 * UH-232 UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. This cau ... References {{Dopaminergics Dopamine agonists Serotonin receptor agonists 2-Aminoindanes Phenol ethers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RU-28306
RU-28306 is a tricyclic tryptamine derivative which acts as a serotonin receptor agonist, with selectivity for 5-HT1 and 5-HT2 subtypes. It can be regarded either as a conformationally constrained derivative of DMT, or a structurally simplified analogue of LSD, but the binding affinity of racemic RU-28306 is closer to that of DMT, though with relatively higher affinity for 5-HT2 subtypes and lower for 5-HT1. It has been sold as a designer drug and was first reported to the EMCDDA by a forensic laboratory in Slovenia in 2017. See also * 4,5-DHP-DMT * Bay R 1531 Bay R 1531 is a tricyclic tryptamine derivative which acts as a selective serotonin receptor 5-HT1A agonist. It was researched unsuccessfully for the treatment of stroke but remains in use for scientific research. See also * 8-OH-DPAT * RDS- ... * NDTDI * RU-24,969 References {{Tryptamines Serotonin receptor agonists Tryptamines Phenethylamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LY-293,284
LY-293284 is a research chemical developed by the pharmaceutical company Eli Lilly and used for scientific studies. It acts as a potent and selective 5-HT1A receptor full agonist. It was derived through structural simplification of the ergoline based psychedelic LSD, but is far more selective for 5-HT1A with over 1000x selectivity over other serotonin receptor subtypes and other targets. It has anxiogenic effects in animal studies. See also * 8-OH-DPAT * RDS-127 * RU-28306 RU-28306 is a tricyclic tryptamine derivative which acts as a serotonin receptor agonist, with selectivity for 5-HT1 and 5-HT2 subtypes. It can be regarded either as a conformationally constrained derivative of DMT, or a structurally simplifi ... References Serotonin receptor agonists Anxiogenics Eli Lilly and Company brands Tryptamines Ketones {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NDTDI
NDTDI is a tricyclic tryptamine derivative which is thought to act as a serotonin receptor agonist, though its pharmacology has not been studied in detail. It is a structurally simplified analogue of LSD and is reported to retain similar effects, though with many times lower potency. It has been sold as a designer drug since 2016 and was first identified by a forensic laboratory in Slovenia in 2017. Legality NDTDI was made illegal in Latvia in March 2017. See also * 4,5-DHP-DMT * Bay R 1531 * RU-28306 RU-28306 is a tricyclic tryptamine derivative which acts as a serotonin receptor agonist, with selectivity for 5-HT1 and 5-HT2 subtypes. It can be regarded either as a conformationally constrained derivative of DMT, or a structurally simplifi ... * RU-24,969 References {{Tryptamines Serotonin receptor agonists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor Agonists
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |