|
BPS Domain
In molecular biology, the BPS domain (Between PH and SH2) domain is a protein domain of approximately 45 amino acids found in the adaptor proteins Grb7/, Grb10/Grb14. It mediates inhibition of the tyrosine kinase domain of the insulin receptor by binding of the N-terminal portion of the BPS domain to the substrate peptide groove of the kinase, acting as a pseudosubstrate inhibitor. It is composed of two beta strands Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labio ... and a C-terminal helix. References Protein domains {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Transducing Adaptor Protein
Signal transducing adaptor proteins (STAPs) are proteins that are accessory to main proteins in a signal transduction pathway. Adaptor proteins contain a variety of protein-binding modules that link protein-binding partners together and facilitate the creation of larger signaling complexes. These proteins tend to lack any intrinsic enzymatic activity themselves, instead mediating specific protein–protein interactions that drive the formation of protein complexes. Examples of adaptor proteins include MYD88, Grb2 and SHC1. Signaling components Much of the specificity of signal transduction depends on the recruitment of several signalling components such as protein kinases and G-protein GTPases into short-lived active complexes in response to an activating signal such as a growth factor binding to its receptor. Domains Adaptor proteins usually contain several domains within their structure (e.g., Src homology 2 (SH2) and SH3 domains) that allow specific interactions with se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRB7
Growth factor receptor-bound protein 7, also known as GRB7, is a protein that in humans is encoded by the ''GRB7'' gene. Function The product of this gene belongs to a small family of adaptor proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with epidermal growth factor receptor (EGFR) and ephrin receptors. The protein plays a role in the integrin signaling pathway and cell migration by binding with focal adhesion kinase (FAK). Alternative splicing results in multiple transcript variants encoding different isoforms, although the full-length natures of only two of the variants have been determined to date. Clinical significance GRB7 is an SH2-domain adaptor protein that binds to receptor tyrosine kinases and provides the intra-cellular direct link to the Ras proto-oncogene. Human GRB7 is located on the long arm of chromosome 17, next to the ERBB ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRB10
Growth factor receptor-bound protein 10 also known as insulin receptor-binding protein Grb-IR is a protein that in humans is encoded by the ''GRB10'' gene. Function The product of this gene belongs to a small family of adaptor proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with insulin receptors and insulin-like growth-factor receptors (e.g., IGF1R and IGF2R). Overexpression of some isoforms of the encoded protein inhibits tyrosine kinase activity and results in growth suppression. This gene is imprinted in a highly isoform- and tissue-specific manner. Alternatively spliced transcript variants encoding different isoforms have been identified. Animal studies Mice whose paternally inherited Grb10 gene is inactivated are more aggressive while those whose maternally inherited allele is inactivated exhibit foetal overgrowth and are significantly bigger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRB14
Growth factor receptor-bound protein 14 is a protein that in humans is encoded by the ''GRB14'' gene. The product of this gene belongs to a small family of adapter proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with insulin receptors and insulin-like growth-factor receptors. This protein likely has an inhibitory effect on receptor tyrosine kinase signaling and, in particular, on insulin receptor signaling. This gene may play a role in signaling pathways that regulate growth and metabolism. Transcript variants have been reported for this gene, but their full-length natures have not been determined to date. Interactions GRB14 has been shown to interact with Epidermal growth factor receptor, Fibroblast growth factor receptor 1 and TNKS2 Tankyrase-2 is an enzyme that in humans is encoded by the ''TNKS2'' gene. Interactions TNKS2 has been shown to intera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
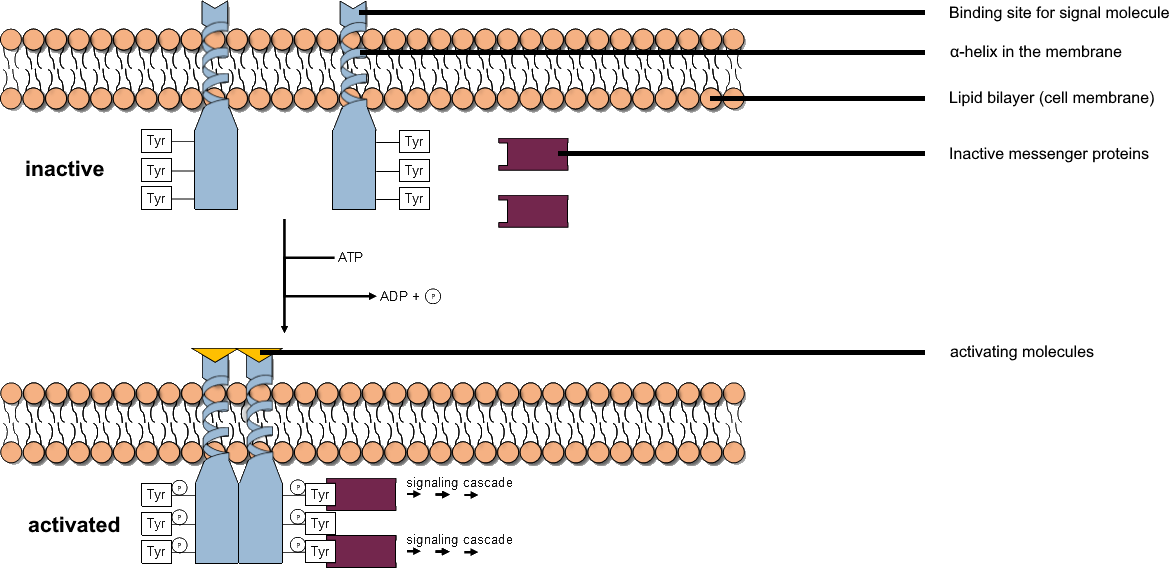
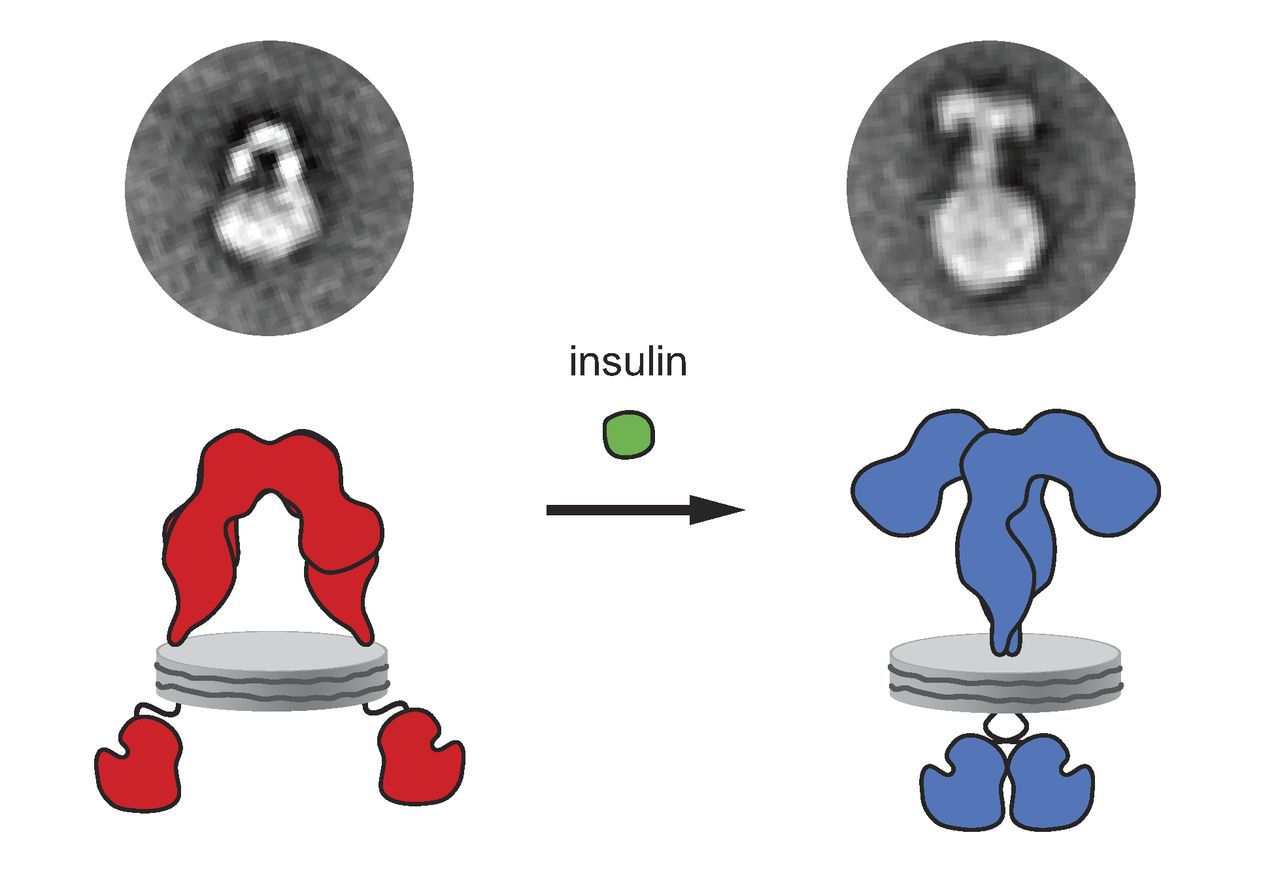
Insulin Receptor
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis, a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene , from which alternate splicing during transcription results in either IR-A or IR-B isoforms. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding (molecular)
Molecular binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It is formed when atoms or molecules bind together by sharing of electrons. It often, but not always, involves some chemical bonding. In some cases, the associations can be quite strong—for example, the protein streptavidin and the vitamin biotin have a dissociation constant (reflecting the ratio between bound and free biotin) on the order of 10−14—and so the reactions are effectively irreversible. The result of molecular binding is sometimes the formation of a molecular complex in which the attractive forces holding the components together are generally non-covalent, and thus are normally energetically weaker than covalent bonds. Molecular binding occurs in biological complexes (e.g., between pairs or sets of proteins, or between a protein and a small molecule ligand it binds) and also in abiologic chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Substrate
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudosubstrate
A decoy (derived from the Dutch ''de'' ''kooi'', literally "the cage" or possibly ''ende kooi'', " duck cage") is usually a person, device, or event which resembles what an individual or a group might be looking for, but it is only meant to lure them. Decoys have been used for centuries most notably in game hunting, but also in wartime and in the committing or resolving of crimes. Hunting In hunting wildfowl, the term decoy may refer to two distinct devices. One, the duck decoy (structure), is a long cone-shaped wickerwork tunnel installed on a small pond to catch wild ducks. After the ducks settled on the pond, a small, trained dog would herd the birds into the tunnel. The catch was formerly sent to market for food, but now these are used only by ornithologists to catch ducks to be ringed and released. The word ''decoy'', also originally found in English as "coy", derives from the Dutch ''de Kooi'' (the cage) and dates back to the early 17th century, when this type of duck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |