|
Azeotropic Distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous (e.g. producing two, immiscible liquid phases), such as the example below with the addition of benzene to water and ethanol. This practice of adding an entrainer which forms a separate phase is a specific sub-set of (industrial) azeotropic distillation methods, or combination thereof. In some senses, adding an entrainer is similar to extractive distillation. Material separation agent The addition of a material separation agent, such as benzene to an ethanol/water mixture, changes the molecular interactions and eliminates the azeotrope. Added in the liquid phase, the new component can alter the activity coefficient of various compounds in different ways thus altering a mixture's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptane
Heptane or ''n''-heptane is the straight-chain alkane with the chemical formula H3C(CH2)5CH3 or C7H16. When used as a test fuel component in anti-knock test engines, a 100% heptane fuel is the zero point of the octane rating scale (the 100 point is 100% iso-octane). Octane number equates to the anti-knock qualities of a comparison mixture of heptane and isooctane which is expressed as the percentage of isooctane in heptane and is listed on pumps for gasoline (petrol) dispensed globally. Uses Heptane (and its many isomers) is widely used in laboratories as a non-polar solvent. As a liquid, it is ideal for transport and storage. In the grease spot test, heptane is used to dissolve an oil spot to show the previous presence of organic compounds on a stained paper. This is done by shaking the stained paper in a heptane solution for about half a minute. Aqueous bromine may be distinguished from aqueous iodine by its appearance after extraction into heptane. In water, both bromine an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theoretical Plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process. Applications The concept of theoretical plates and trays or equilibrium stages is used in the design of many different types of separation. Distillation columns The concept of theoretical plates in designing distillation processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
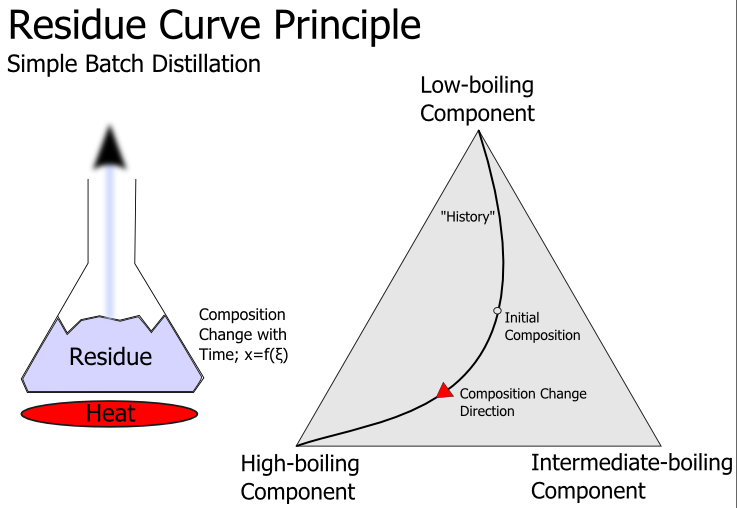
Residue Curve
A residue curve describes the change of the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium (open distillation). Multiple residue curves for a single system are called ''residue curves map''. Residue curves allow testing the feasibility of a separation of mixtures and therefore are a valuable tool in designing distillation processes. Residue curve maps are typically used for examining ternary mixtures which can't be easily separated by distillation because of azeotropic points or too small relative volatilities. Characteristics # Residue curves start at the composition of a feed and then move to pure components or azeotropic points with higher temperatures (isobaric condition) or lower vapor pressures (isothermal condition). This happens because more of the light boiling substances are vaporized than of the high boiling substances and therefore the concentration of the high boilers increase in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azeotrope Tables
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight (in binary azeotropes, when only one fraction is given, it is the fraction of the second component), the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers. The data were obtained from Lange's 10th edition''Lange's Handbook of Chemistry'', 10th ed. pp1496-1505 and ''CRC Handbook of Chemistry and Physics'' 44th edition''CRC Handbook of Chemistry and Physics'', 44th ed. pp 2143-2184 unless otherwise noted (see color code table). A list of 15825 binary and ternary mixtures was collated and published by the American Chemical Society. An azeotrope databank is also available online through the University ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
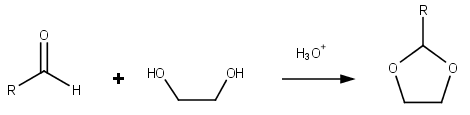
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls including as dioxolanes are known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Oven
A vacuum furnace is a type of furnace in which the product in the furnace is surrounded by a vacuum during processing. The absence of air or other gases prevents oxidation, heat loss from the product through convection, and removes a source of contamination. This enables the furnace to heat materials (typically metals and ceramics) to temperatures as high as with select materials. Maximum furnace temperatures and vacuum levels depend on melting points and vapor pressures of heated materials. Vacuum furnaces are used to carry out processes such as annealing, brazing, sintering and heat treatment with high consistency and low contamination. Characteristics of a vacuum furnace are: * Uniform temperatures in the range. * Commercially available vacuum pumping systems can reach vacuum levels as low as * Temperature can be controlled within a heated zone, typically surrounded by heat shielding or insulation. * Low contamination of the product by carbon, oxygen and other gases. * Va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Sieve
A molecular sieve is a material with pores (very small holes) of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrate through the stationary bed of porous, semi-solid substance referred to as a sieve (or matrix), the components of highest molecular weight (which are unable to pass into the molecular pores) leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in size-exclusion chromatography, a separation technique that sorts molecules based on their size. Other molecular sieves are used as desiccants (some examples include activated charcoal and silica gel). The pore diameter of a molecular sieve is measured in ångströms (Å) or nanometres (nm). According to IUPAC notation, microporous materials have pore diameters of less than 2 nm (20 Å) and macroporous materials have pore diameters of gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
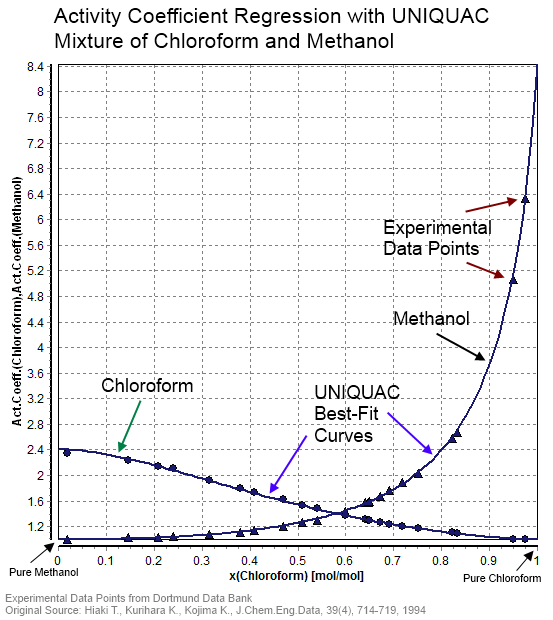
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\mu_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm. History The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it ''rétinnaphte''. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree ''Myroxylon balsamum''), which Deville recognized as similar to Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |