|
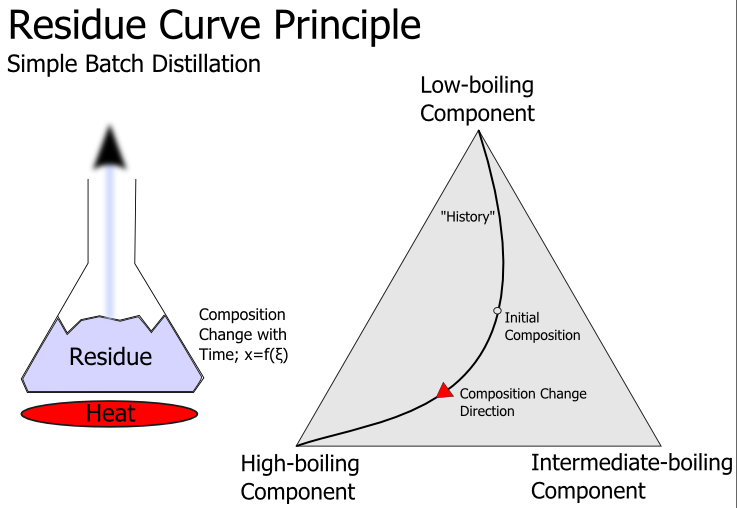
Residue Curve
A residue curve describes the change of the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium (open distillation). Multiple residue curves for a single system are called ''residue curves map''. Residue curves allow testing the feasibility of a separation of mixtures and therefore are a valuable tool in designing distillation processes. Residue curve maps are typically used for examining ternary mixtures which can't be easily separated by distillation because of azeotropic points or too small relative volatilities. Characteristics # Residue curves start at the composition of a feed and then move to pure components or azeotropic points with higher temperatures (isobaric condition) or lower vapor pressures (isothermal condition). This happens because more of the light boiling substances are vaporized than of the high boiling substances and therefore the concentration of the high boilers increase in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
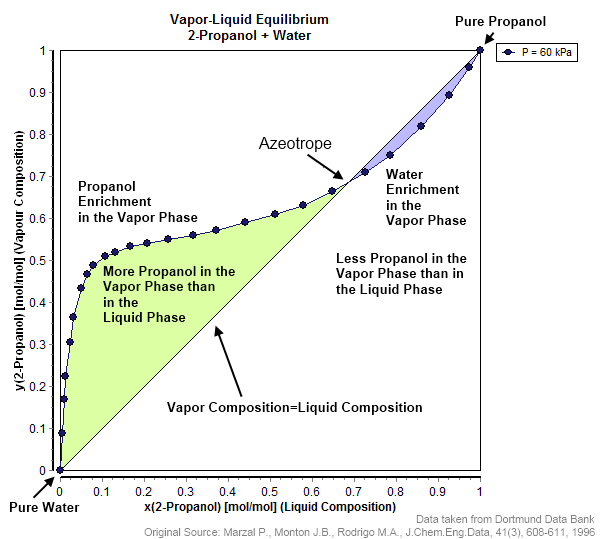
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) ''constant boiling point mixtures''. Some azeotropic mixtures of pairs of compounds are known, and many azeotropes of three or more compounds are also known. In such a case it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. There are two types of azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A Solution (chemistry), solution that shows greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relative Volatility
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as \alpha. Relative volatilities are used in the design of all types of distillation processes as well as other separation or absorption processes that involve the contacting of vapor and liquid phases in a series of equilibrium stages. Relative volatilities are not used in separation or absorption processes that involve components reacting with each other (for example, the absorption of gaseous carbon dioxide in aqueous solutions of sodium hydroxide). Definition For a liquid mixture of two components (called a ''binary mixture'') at a given temperature and pressure, the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curve
In mathematics, a curve (also called a curved line in older texts) is an object similar to a line (geometry), line, but that does not have to be Linearity, straight. Intuitively, a curve may be thought of as the trace left by a moving point (geometry), point. This is the definition that appeared more than 2000 years ago in Euclid's Elements, Euclid's ''Elements'': "The [curved] line is […] the first species of quantity, which has only one dimension, namely length, without any width nor depth, and is nothing else than the flow or run of the point which […] will leave from its imaginary moving some vestige in length, exempt of any width." This definition of a curve has been formalized in modern mathematics as: ''A curve is the image (mathematics), image of an interval (mathematics), interval to a topological space by a continuous function''. In some contexts, the function that defines the curve is called a ''parametrization'', and the curve is a parametric curve. In this artic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Runge–Kutta Methods
In numerical analysis, the Runge–Kutta methods ( ) are a family of implicit and explicit iterative methods, which include the Euler method, used in temporal discretization for the approximate solutions of simultaneous nonlinear equations. These methods were developed around 1900 by the German mathematicians Carl Runge and Wilhelm Kutta. The Runge–Kutta method The most widely known member of the Runge–Kutta family is generally referred to as "RK4", the "classic Runge–Kutta method" or simply as "the Runge–Kutta method". Let an initial value problem be specified as follows: : \frac = f(t, y), \quad y(t_0) = y_0. Here y is an unknown function (scalar or vector) of time t, which we would like to approximate; we are told that \frac, the rate at which y changes, is a function of t and of y itself. At the initial time t_0 the corresponding y value is y_0. The function f and the initial conditions t_0, y_0 are given. Now we pick a step-size ''h'' > 0 and define: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jürgen Gmehling
Jürgen Gmehling (born January 13, 1946 in Duisburg) is a retired German professor of technical and industrial chemistry at the Carl von Ossietzky University of Oldenburg. Biography His career started with an apprenticeship as a laboratory assistant at the Duisburg copper works before he studied chemical engineering at the engineering school in Essen and then chemistry in Dortmund and Clausthal. He received his diploma from the University of Dortmund in 1970 and his PhD (Dr. rer. nat., inorganic chemistry) in 1973. After this he worked as a scientific coworker in Dortmund before he became a private lecturer and, after his habilitation, an assistant professor. Gmehling was appointed a full professor for technical chemistry at the University of Oldenburg in 1989 and retired in 2011. Fields of research Gmehling's main focus is the process development. This includes the development of software for process synthesis and process simulation as well as measurement, collection, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |