|
A·h
An ampere hour or amp hour (symbol: A⋅h or A h; often simplified as Ah) is a Unit of measurement, unit of electric charge, having Dimensional analysis, dimensions of electric current multiplied by time, equal to the charge transferred by a steady current of one ampere flowing for one hour, or 3,600 coulombs. The commonly seen milliampere hour (symbol: mA⋅h, mA h, simplified as mAh) is one-thousandth of an ampere hour (3.6 coulombs). Use The ampere hour is frequently used in measurements of electrochemistry, electrochemical systems such as electroplating and for battery capacity where the commonly known Real versus nominal value, nominal voltage is dropped. A ''milliampere second'' (mA⋅s) is a unit of measurement used in X-ray imaging, diagnostic imaging, and radiation therapy. It is equivalent to a ''millicoulomb''. This quantity is proportional to the total X-ray energy produced by a given X-ray tube operated at a particular voltage. The same total dose can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Capacity
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons that will flow through an external electric circuit to the positive terminal. When a battery is connected to an external electric load, a redox reaction converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell. Primary (single-use or "disposable") batteries are used once and discarded, as the electrode materials are irreversibly changed during discharge; a common example is the alkaline battery used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faraday Constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of SI base units, which took effect on 20 May 2019, the Faraday constant has the exactly defined value given by the product of the elementary charge ''e'' and Avogadro constant ''N''A: : : :. Derivation The Faraday constant can be thought of as the conversion factor between the mole (used in chemistry) and the coulomb (used in physics and in practical electrical measurements), and is therefore of particular use in electrochemistry. Because 1 mole contains exactly entities, and 1 coulomb contains exactly elementary charges, the Faraday constant is given by the quotient of these two quantities: :. One common use of the Faraday constant is in electrolysis calculations. One can divide the amount of charge (the current integrated over t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Car Battery
An automotive battery or car battery is a rechargeable battery that is used to start a motor vehicle. Its main purpose is to provide an electric current to the electric-powered starting motor, which in turn starts the chemically-powered internal combustion engine that actually propels the vehicle. Once the engine is running, power for the car's electrical systems is still supplied by the battery, with the alternator charging the battery as demands increase or decrease. Battery in modern cars Gasoline and diesel engine Typically, starting uses less than three percent of the battery capacity. For this reason, automotive batteries are designed to deliver maximum current for a short period of time. They are sometimes referred to as "SLI batteries" for this reason, for starting, lighting and ignition. SLI batteries are not designed for deep discharging, and a full discharge can reduce the battery's lifespan. As well as starting the engine, an SLI battery supplies the extra power nec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects. Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Imaging
Radiography is an imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical radiography ("diagnostic" and "therapeutic") and industrial radiography. Similar techniques are used in airport security (where "body scanners" generally use backscatter X-ray). To create an image in conventional radiography, a beam of X-rays is produced by an X-ray generator and is projected toward the object. A certain amount of the X-rays or other radiation is absorbed by the object, dependent on the object's density and structural composition. The X-rays that pass through the object are captured behind the object by a detector (either photographic film or a digital detector). The generation of flat two dimensional images by this technique is called projectional radiography. In computed tomography (CT scanning) an X-ray source and its associated detectors rotate around the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilowatt-hour
A kilowatt-hour ( unit symbol: kW⋅h or kW h; commonly written as kWh) is a unit of energy: one kilowatt of power for one hour. In terms of SI derived units with special names, it equals 3.6 megajoules (MJ). Kilowatt-hours are a common billing unit for electrical energy delivered to consumers by electric utilities. Definition The kilowatt-hour is a composite unit of energy equal to one kilowatt (kW) sustained for (multiplied by) one hour. Expressed in the standard unit of energy in the International System of Units (SI), the joule (symbol J), it is equal to 3,600 kilojoules or 3.6 MJ."Half-high dots or spaces are used to express a derived unit formed from two or more other units by multiplication.", Barry N. Taylor. (2001 ed.''The International System of Units.'' (Special publication 330). Gaithersburg, MD: National Institute of Standards and Technology. 20. Unit representations A widely used representation of the kilowatt-hour is "kWh", derived from its com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Equivalent
The Electrochemical equivalent, sometimes abbreviated Eq or Z, of a chemical element is the mass of that element (in grams) transported by 1 coulomb of electric charge. The electrochemical equivalent of an element is measured with a voltameter A voltameter or coulometer is a scientific instrument used for measuring electric charge (quantity of electricity) through electrolytic action. The SI unit of electric charge is the coulomb. The voltameter should not be confused with a voltmete .... Definition The electrochemical equivalent of a substance is the mass of the substance deposited to one of the electrodes when a current of 1 ampere is passed for 1 second, i.e. a quantity of electricity of one coulomb is passed. The formula for finding electrochemical equivalent is as follows: :Z = M/q where M is the mass of substance and q is the charge passed. Since q=It, where I is the current applied and t is time, we also have :Z=M/It Eq values of some elements in kg/C Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cell
upLine art drawing of a dry cell: 1. brass cap, 2. plastic seal, 3. expansion space, 4. porous cardboard, 5. zinc can, 6. carbon rod, 7. chemical mixture A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry cells use an electrolyte in the form of a paste, and are thus less susceptible to leakage. The dry cell was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc–carbon batteries by Georges Leclanché in 1866. A type of dry cell was also developed by the Japanese Sakizō Yai in 1887. History left, upDry cell battery by Wilhelm Hellesen 1890 Many experimenters tried to immobilize the electrolyte of an electrochemical cell to make it more convenient to use. The Zamboni pile of 1812 is a high-voltage dry battery but capable of delivering only minute currents. Various experiments were made with cellulose, sawdust, spun glass, asbestos fib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Battery Sizes
This is a list of the sizes, shapes, and general characteristics of some common primary and secondary battery types in household, automotive and light industrial use. The complete nomenclature for a battery specifies size, chemistry, terminal arrangement, and special characteristics. The same physically interchangeable cell size or battery size may have widely different characteristics; physical interchangeability is not the sole factor in substituting a battery. The full battery designation identifies not only the size, shape and terminal layout of the battery but also the chemistry (and therefore the voltage per cell) and the number of cells in the battery. For example, a CR123 battery is always LiMnO2 ('Lithium') chemistry, in addition to its unique size. The following tables give the common battery chemistry types for the current common sizes of batteries. See Battery chemistry for a list of other electrochemical systems. Cylindrical batteries Rectangular batteries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Units Of Energy
Energy is defined via work, so the SI unit of energy is the same as the unit of work – the joule (J), named in honour of James Prescott Joule and his experiments on the mechanical equivalent of heat. In slightly more fundamental terms, is equal to 1 newton metre and, in terms of SI base units :1\ \mathrm = 1\ \mathrm \left( \frac \right ) ^ 2 = 1\ \frac An energy unit that is used in atomic physics, particle physics and high energy physics is the electronvolt (eV). One eV is equivalent to . In spectroscopy the unit cm−1 ≈ is used to represent energy since energy is inversely proportional to wavelength from the equation E = h \nu = h c/\lambda . In discussions of energy production and consumption, the units barrel of oil equivalent and ton of oil equivalent are often used. British imperial / US customary units The British imperial units and U.S. customary units for both energy and work include the foot-pound force (1.3558 J), the British ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
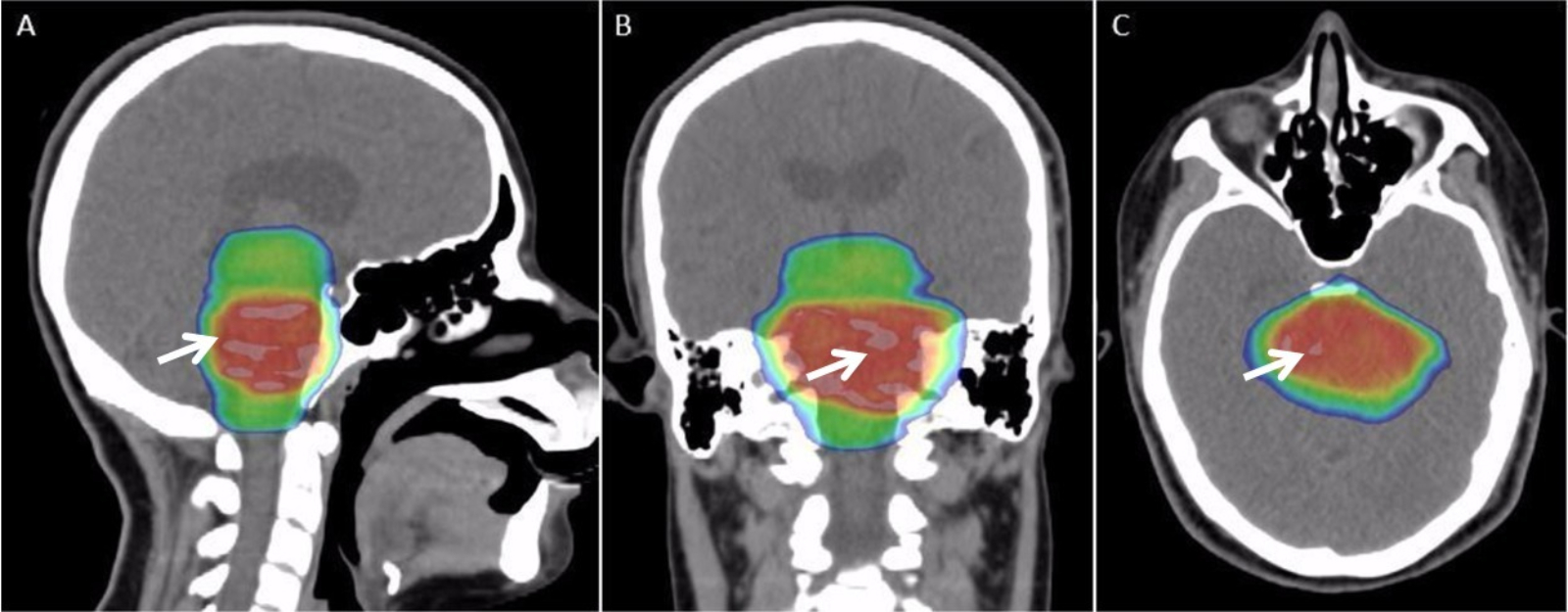
Radiation Therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizing radiation works by damaging the DNA of cancerous tissue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)