|
Azide-alkyne Huisgen Cycloaddition
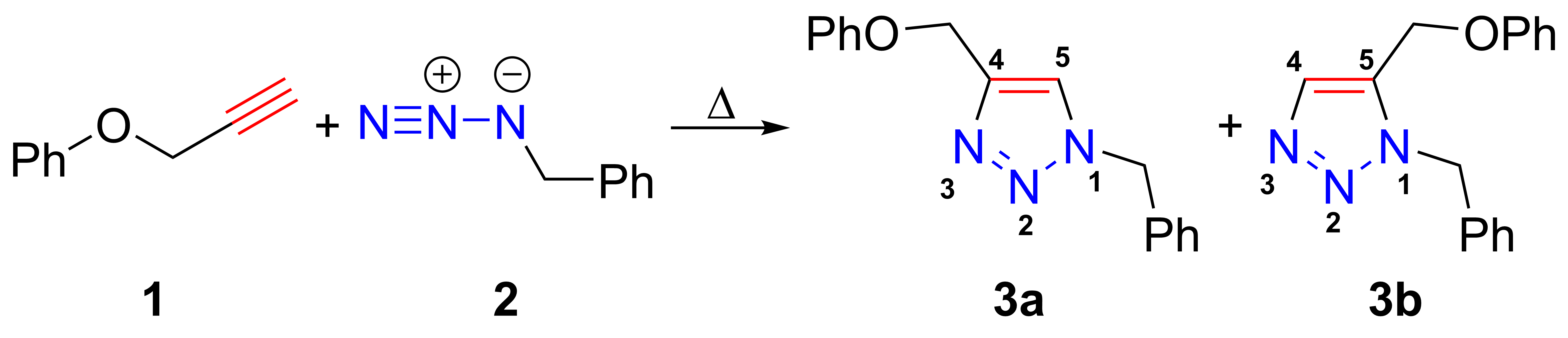
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rolf Huisgen
Rolf Huisgen (; 13 June 1920 – 26 March 2020) was a German chemist. His importance in synthetic organic chemistry extends to the enormous influence he had in post-war chemistry departments in Germany and Austria, due to a large number of his habilitants becoming professors. His major achievement was the development of the 1,3-dipolar cycloaddition reaction, also called the Huisgen cycloaddition. Life Huisgen was born in Gerolstein in Rhineland-Palatinate and studied in Munich under the supervision of Heinrich Otto Wieland. He completed his Ph.D. in 1943 with a thesis about a strychnine alkaloid. He completed his habilitation in 1947, and was appointed professor at the University of Tübingen in 1949. He returned to the University of Munich in 1952, succeeding Wieland, and he remained dedicated to research long after attaining emeritus status there in 1988. One of his major achievements was the development of the 1,3-dipolar cycloaddition reaction, also known as the Huisg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Banert Cascade
The Banert cascade is an organic reaction in which an NH-1,2,3-triazole is prepared from a propargyl halide or sulfate and sodium azide in a dioxane- water mixture at elevated temperatures. It is named after Klaus Banert, who first reported the process in 1989. This cascade reaction is unusual because it consists of two consecutive rearrangement reactions. The starting material is prepared from propargyl chloride and an aldehyde or ketone such as acetaldehyde. In the first step an azido compound is formed in situ in a nucleophilic displacement of chloride by the azide ion. A (3,3)Sigmatropic reaction takes place between the azide and the alkyne to the allenyl azide. This allene rearranges to the triazafulvene in a 6 pi electrocyclization. The exocyclic alkene in this intermediate is very electrophilic because the triazole group has a dipole moment of 5 debye. The reaction sequence concludes with nucleophilic attack In chemistry, a nucleophile is a chemical species that forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free-radical Polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain. Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization. Free-radical polymerization is a type of chain-growth polymeriza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Australian Journal Of Chemistry
The ''Australian Journal of Chemistry - an International Journal for Chemical Science'' is a monthly peer-reviewed scientific journal published by CSIRO Publishing. It was established in 1948 and covers all aspects of chemistry. The editors-in-chief are George Koutsantonis (University of Western Australia) and John Wade (University of Melbourne). Abstracting and indexing The journal is abstracted and indexed in: *CAB Abstracts *Chemical Abstracts Service *Current Contents/Physical, Chemical & Earth Sciences *EBSCO databases *Ei Compendex *Science Citation Index *Scopus According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 1.32. See also * ''Environmental Chemistry'' (journal) * List of scientific j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
End-group
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, they are functional groups that are at the very ends of a macromolecule or oligomer ( IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed by nuclear magnetic resonance ( NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and raman spectroscopy. These groups are important for the analysis of polymers and for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled. End groups in polymer synthesis End groups are seen on all polymers and the functionality of those end groups can be important in determining the applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Azide
Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group. The C−N=N angle is approximately 120°. Preparation Phenyl azide is prepared by the diazotization of phenylhydrazine with nitrous acid: :C6H5NHNH2 + HNO2 → C6H5N3 + 2 H2O Aryl iodides bearing electron-withdrawing substituents undergo metathesis with sodium azide in the presence of Cu(I), sodium ascorbate, and N,N'-dimethylethane-1,2-diamine (DMEDA): :RC6H4I + NaN3 → RC6H4N3 + NaI It can also be prepared by condensation of benzenediazonium salt with toluenesulfonamide, followed by hydrolysis. Chemical reactions Phenyl azide cycloadds to alkenes and especially alkynes, particularly those bearing electronegative substituents. In a classic example of click chemistry, phenyl azide and phenylacetylene react to give diphenyl t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
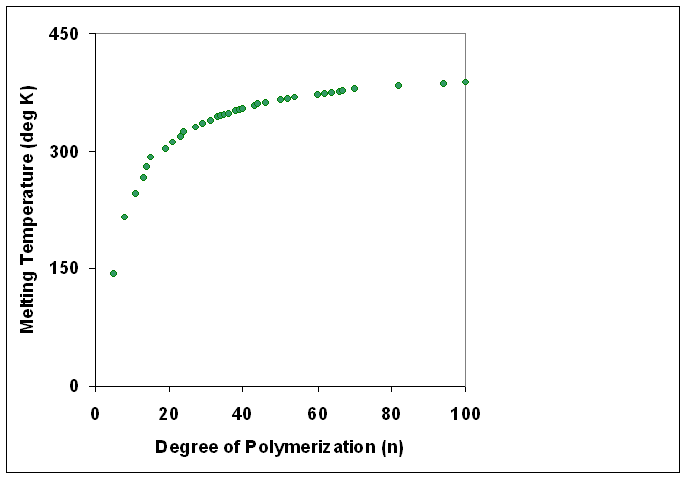
Degree Of Polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by DP_n\equiv X_n=\frac, where Mn is the number-average molecular weight and M0 is the molecular weight of the monomer unit. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units. Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27 For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO—, so that a chain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Communications
''ChemComm'' (or ''Chemical Communications''), formerly known as ''Journal of the Chemical Society D: Chemical Communications'' (1969–1971), ''Journal of the Chemical Society, Chemical Communications'' (1972–1995), is a peer-reviewed scientific journal published by the Royal Society of Chemistry. It covers all aspects of chemistry. In January 2012, the journal moved to publishing 100 issues per year. The current chair of the Editorial Board is Douglas Stephan (University of Toronto, Canada), while the executive editor is Richard Kelly. Abstracting and indexing The journal is abstracted and indexed in: * Chemical Abstracts * Science Citation Index * Current Contents/Physical, Chemical & Earth Sciences * Scopus * Index Medicus/MEDLINE/PubMed According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 6.065. See also * '' New Journal of Chemistry'' * '' Chemical Society Reviews'' * '' Chemical Science'' * ''RSC Advances ''RSC Advances'' is an onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |