|
Auric Acetate
Gold(III) acetate, also known as auric acetate, is a chemical compound of gold and acetic acid. It is a yellow solid that decomposes at 170 °C to gold metal. This decomposition of gold(III) acetate has been studied as a pathway to produce gold nanoparticles as catalysts. Production and reactions Gold(III) acetate can be produced by the reaction of gold(III) hydroxide and glacial acetic acid: :Au(OH)3 + 3CH3COOH → Au(CH3COO)3 + 3H2O It reacts with 2-(''p''-tolyl)pyridine (tpy) in presence of trifluoroacetic acid to form Au(CF3COO)2(tpy). Gold(III) sulfide has been claimed as the product when gold(III) acetate is sonicated with ''cyclo''-octasulfur in decalin Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. I .... References {{Acetates Gold(III) compounds Acetates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
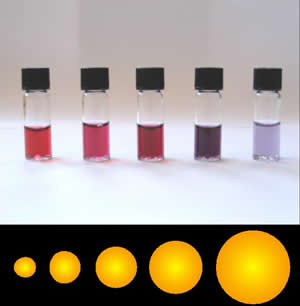
Gold Nanoparticles
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either wine-red coloured (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass colloidal gold was used in the 4th-century Lycurgus Cup, which changes color depending on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold(III) Hydroxide
Gold(III) hydroxide, gold trihydroxide, or gold hydroxide is an inorganic compound, a hydroxide of gold, with formula Au(OH)3. It is also called auric acid with formula H3AuO3. It is easily dehydrated above 140 °C to gold(III) oxide. Salts of auric acid are termed aurates. Gold hydroxide is used in medicine, porcelain making, gold plating, and daguerrotypes. Gold hydroxide deposited on suitable carriers can be used for preparation of gold catalysts. Gold hydroxide is a product of electrochemical corrosion of gold metalization subjected to moisture and positive electric potential; it is one of the corrosion failure modes of microelectronics. Voluminous gold hydroxide is produced from gold metalization; after the layer grows thick it may spall, and the conductive particles may cause short circuits or leakage paths. The decreased thickness of the gold layer may also lead to an increase in its electrical resistance, which can also lead to electrical failure. Preparation and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold(III) Sulfide
Gold(III) sulfide or auric sulfide is an inorganic compound with the formula .Auric sulfide has been described as a black and amorphous. Little evidence has been published supporting the existence of macroscopic quantities of this material. Claims Early investigations claimed to prepare auric sulfide by the reaction of lithium tetrachloroaurate with hydrogen sulfide: : Similar preparations via chloroauric acid, auric chloride, or gold(III) sulfate a claimed proceed in anhydrous solvents, but water evinces a redox decomposition into metallic gold in sulfuric acid: : Conversely, ''cyclo''-octasulfur reduces gold(III) sulfate to a mixture of gold sulfides and sulfur oxides: : Auric sulfide has also been claimed as the product when auric acetate is sonicated with ''cyclo''-octasulfur in decalin. Auric sulfide is claimed to react with nitric acid as well sodium cyanide. It is claimed to dissolve in concentrated sodium sulfide solution. See also * Gold(I) sulfide Gold(I) sulfi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decalin
Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. Isomers Decalin occurs in ''cis'' and ''trans'' forms. The ''trans'' form is energetically more stable because of fewer steric interactions. ''cis''-Decalin is a chiral molecule without a chiral center; it has a two-fold rotational symmetry axis, but no reflective symmetry. However, the chirality is canceled through a chair-flipping process that turns the molecule into its mirror image. Image:Cis-trans isomerism of decahydronaphthalene.svg, Image:cis-decalin double chair.png, 2: Image:trans-decalin double chair.png, 3: File:Cisdecalin conformations.png, 4: ''trans''-Decalin The only possible way to join the two six-membered rings in the ''trans'' position means the second ring needs to start from two equatorial bonds (blue) of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold(III) Compounds
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in a pure form. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements and is solid under standard conditions. Gold often occurs in free elemental (native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides). Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroaurate anion. Gold is i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |