|
Gold(III) Hydroxide
Gold(III) hydroxide, gold trihydroxide, or gold hydroxide is an inorganic compound, a hydroxide of gold, with formula Au(OH)3. It is also called auric acid with formula H3AuO3. It is easily dehydrated above 140 °C to gold(III) oxide. Salts of auric acid are termed aurates. Gold hydroxide is used in medicine, porcelain making, gold plating, and daguerrotypes. Gold hydroxide deposited on suitable carriers can be used for preparation of gold catalysts. Gold hydroxide is a product of electrochemical corrosion of gold metalization subjected to moisture and positive electric potential; it is one of the corrosion failure modes of microelectronics. Voluminous gold hydroxide is produced from gold metalization; after the layer grows thick it may spall, and the conductive particles may cause short circuits or leakage paths. The decreased thickness of the gold layer may also lead to an increase in its electrical resistance, which can also lead to electrical failure. Preparation and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold(III) Chloride
Gold(III) chloride, traditionally called auric chloride, is a compound of gold and chlorine with the molecular formula . The "III" in the name indicates that the gold has an oxidation state of +3, typical for many gold compounds. Gold(III) chloride is hygroscopic and decomposes in visible light. This compound is a dimer of . This compound has few uses, although it catalyzes various organic reactions. Structure exists as a chloride-bridged dimer both as a solid and vapour, at least at low temperatures. Gold(III) bromide behaves analogously. The structure is similar to that of iodine(III) chloride. Each gold center is square planar in gold(III) chloride, which is typical of a metal complex with a d8 electron count. The bonding in is considered somewhat covalent. Preparation Gold(III) chloride is most often prepared by passing chlorine gas over gold powder at : : The chlorination reaction can be conducted in the presence of tetrabutylammonium chloride, the product bein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fulminating Gold
Fulminating gold is a light- and shock-sensitive yellow to yellow-orange amorphous heterogeneous mixture of different polymeric compounds of predominantly gold (III), ammonia, and chlorine that cannot be described by a chemical formula. Here, the word fulminating has its oldest meaning, "explosive" (from Latin fulmen, lightning, from verb fulgeo, 'I shine'); the material contains no fulminate ions. The best approximate description is that it is the product of partial hydrolysis of ^3_\infty \ce. Upon combustion, it produces a purple vapor. The complex has a square planar molecular geometry with a low spin state. Generally, it is best to avoid accidentally creating this substance by mixing gold chloride or hydroxide salts with ammonia gas or ammonium salts, as it is prone to explosion with even the slightest touch. History Fulminating gold was the first high explosive known to man and was first noted in western alchemy as early as 1585. Sebald Schwaerzer was the first to isolate thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroauric Acid
Chloroauric acid is an inorganic compound with the chemical formula . It forms hydrates . Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles. Properties Structure The tetrahydrate crystallizes as and two water molecules. The oxidation state of gold in and anion is +3. The salts of (tetrachloroauric(III) acid) are tetrachloroaurates(III), containing anions (tetrachloroaurate(III) anions), which have square planar molecular geometry. The Au–Cl distances are around 2.28 Å. Other d8 complexes adopt similar structures, e.g. tetrachloroplatinate(II) . Solute properties Solid chloroauric acid is a hydrophilic ( ionic) protic solute. It is soluble in water and other oxygen-containing solvents, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spall
Spall are fragments of a material that are broken off a larger solid body. It can be produced by a variety of mechanisms, including as a result of projectile impact, corrosion, weathering, cavitation, or excessive rolling pressure (as in a ball bearing). Spalling and spallation both describe the process of surface failure in which spall is shed. The terms ''spall'', ''spalling'', and ''spallation'' have been adopted by particle physicists; in neutron scattering instruments, neutrons are generated by bombarding a uranium (or other) target with a stream of atoms. The neutrons that are ejected from the target are known as "spall". Mechanical spalling Mechanical spalling occurs at high stress contact points, for example, in a ball bearing. Spalling occurs in preference to brinelling where the maximal shear stress occurs not at the surface, but just below, shearing the spall off. One of the simplest forms of mechanical spalling is plate impact, in which two waves of compression ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
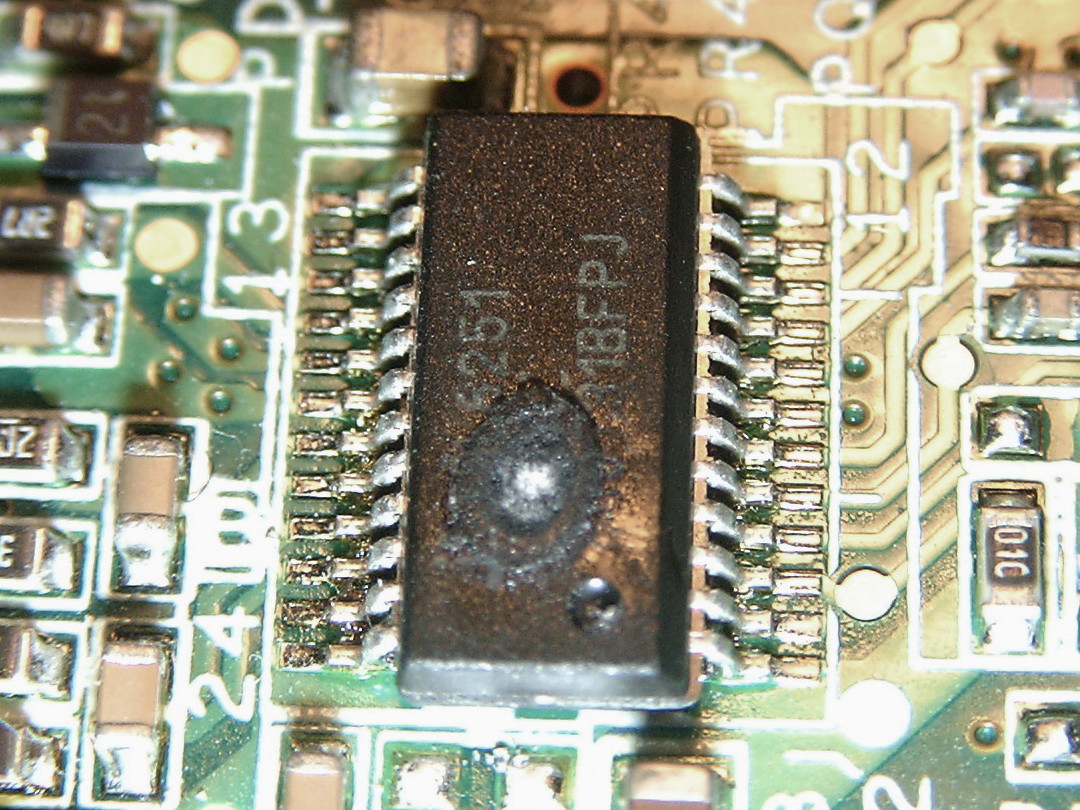
Microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre-scale or smaller. These devices are typically made from semiconductor materials. Many components of normal electronic design are available in a microelectronic equivalent. These include transistors, capacitors, inductors, resistors, diodes and (naturally) insulators and conductors can all be found in microelectronic devices. Unique wiring techniques such as wire bonding are also often used in microelectronics because of the unusually small size of the components, leads and pads. This technique requires specialized equipment and is expensive. Digital integrated circuits (ICs) consist of billions of transistors, resistors, diodes, and capacitors. Analog circuits commonly contain resistors and capacitors as well. Inductors are used in som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Failure Modes Of Electronics
Electronic components have a wide range of failure modes. These can be classified in various ways, such as by time or cause. Failures can be caused by excess temperature, excess current or voltage, ionizing radiation, mechanical shock, stress or impact, and many other causes. In semiconductor devices, problems in the device package may cause failures due to contamination, mechanical stress of the device, or open or short circuits. Failures most commonly occur near the beginning and near the ending of the lifetime of the parts, resulting in the bathtub curve graph of failure rates. Burn-in procedures are used to detect early failures. In semiconductor devices, parasitic structures, irrelevant for normal operation, become important in the context of failures; they can be both a source and protection against failure. Applications such as aerospace systems, life support systems, telecommunications, railway signals, and computers use great numbers of individual electronic componen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daguerrotype
Daguerreotype (; french: daguerréotype) was the first publicly available photographic process; it was widely used during the 1840s and 1850s. "Daguerreotype" also refers to an image created through this process. Invented by Louis Daguerre and introduced worldwide in 1839, the daguerreotype was almost completely superseded by 1860 with new, less expensive processes, such as ambrotype (collodion process), that yield more readily viewable images. There has been a revival of the daguerreotype since the late 20th century by a small number of photographers interested in making artistic use of early photographic processes. To make the image, a daguerreotypist polished a sheet of silver-plated copper to a mirror finish; treated it with fumes that made its surface light-sensitive; exposed it in a camera for as long as was judged to be necessary, which could be as little as a few seconds for brightly sunlit subjects or much longer with less intense lighting; made the resulting laten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Hydroxide
Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly. Occurrence Copper(II) hydroxide has been known since copper smelting began around 5000 BC although the alchemists were probably the first to manufacture it by mixing solutions of lye (sodium or potassium hydroxide) and blue vitriol (copper(II) sulfate). Sources of both compounds were available in antiquity. It was produced on an industrial scale during the 17th and 18th centuries for use in pigments such as blue verditer and Bremen green. These pigments were used in ceramics and painting. Mineral The mineral of the formula Cu(OH)2 is called spertiniite. Copper(II) hydroxide is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold Plating
Gold plating is a method of depositing a thin layer of gold onto the surface of another metal, most often copper or silver (to make silver-gilt), by chemical or electrochemical plating. This article covers plating methods used in the modern electronics industry; for more traditional methods, often used for much larger objects, see gilding. Types There are several types of gold plating used in the electronics industry: * ''Soft, pure gold plating'' is used in the semiconductor industry. The gold layer is easily soldered and wire bonded. Its Knoop hardness ranges between 60 and 85. The plating baths have to be kept free of contamination. * ''Soft, pure gold'' is deposited from special electrolytes. Entire printed circuit boards can be plated. This technology can be used for depositing layers suitable for wire bonding. * ''Bright hard gold on contacts'', with Knoop hardness between 120–300 and purity of 99.7–99.9% gold. Often contains a small amount of nickel and/or cobalt; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_chloride_solution.jpg)