|
Ardisiaquinone
Ardisiaquinones are a group of closely-related chemical compounds found in plants in the genus ''Ardisia''. The first examples, ardisiaquinones A-C, were isolated in 1968 from ''Ardisia sieboldii''. In 1995, ardisiaquinones D, E, and F were discovered, also from ''Ardisia sieboldii''. In 2001, ardisiaquinones G, H and I were isolated from ''Ardisia teysmanniana''. Chemically, the ardisiaquinones consist of two variably-substituted 1,4-benzoquinone units connected by a long alkyl or alkenyl chain. Research Ardisiaquinones are of research interest because they possess 5-lipoxygenase (5-LOX) inhibitor activity and 5-LOX has clinical relevance in inflammation. For example, ardisiaquinone A protects against liver injury in an animal model of ischemia-reperfusion injury. Likewise, ardisiaquinone G has also shown 5-LOX inhibition. Ardisiaquinone A has also been shown to have an antiallergic effect in an animal model. Other ardisiaquinones have shown antiproliferative and antimicro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound. Preparation 1,4-Benzoquinone is prepared industrially by oxidation of hydroquinone, which can be obtained by several routes. One route involves oxidation of diisopropylbenzene and the Hock rearrangement. The net reaction can be represented as follows: :C6H4(CHMe2)2 + 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ardisia
''Ardisia'' (coralberry or marlberry) is a genus of flowering plants in the family Primulaceae. It was in the former Myrsinaceae family now recognised as the myrsine sub-family Myrsinoideae. They are distributed in the Americas, Asia, Australia, and the Pacific Islands, mainly in the tropics.''Ardisia''. Flora of North America.''Ardisia''. Flora of China. There are over 700 accepted species. One species, '''' is one of the 50 fundamental herbs in |
Ardisia Sieboldii
''Ardisia'' (coralberry or marlberry) is a genus of flowering plants in the family Primulaceae. It was in the former Myrsinaceae family now recognised as the myrsine sub-family Myrsinoideae. They are distributed in the Americas, Asia, Australia, and the Pacific Islands, mainly in the tropics.''Ardisia''. Flora of North America.''Ardisia''. Flora of China. There are over 700 accepted species. One species, '''' is one of the 50 fundamental herbs in |
Ardisia Teysmanniana
''Ardisia'' (coralberry or marlberry) is a genus of flowering plants in the family Primulaceae. It was in the former Myrsinaceae family now recognised as the myrsine sub-family Myrsinoideae. They are distributed in the Americas, Asia, Australia, and the Pacific Islands, mainly in the tropics.''Ardisia''. Flora of North America.''Ardisia''. Flora of China. There are over 700 accepted species. One species, '''' is one of the 50 fundamental herbs in |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arachidonate 5-lipoxygenase Inhibitor
Arachidonate 5-lipoxygenase inhibitors are compounds that slow or stop the action of the arachidonate 5-lipoxygenase (5-lipoxygenase or 5-LOX) enzyme, which is responsible for the production of inflammatory leukotrienes. The overproduction of leukotrienes is a major cause of inflammation in asthma, allergic rhinitis, and osteoarthritis. Examples of 5-LOX inhibitors include the pharmaceutical drugs meclofenamate sodium, zileuton and the natural products myxochelins/pseudochelin as well as nordihydroguaiaretic acid (NDGA). Some chemicals found in trace amounts in food, as well as some dietary supplements, have been shown to inhibit 5-LOX; these include baicalein, caffeic acid, curcumin, hyperforin and St John's wort. 1. Arachidonate 5-lipoxygenase ...Specific function: Catalyzes the first step in leukotriene biosynthesis, and thereby plays a role in inflammatory processes ...2. Prostaglandin G/H synthase 1 ... General function: Involved in peroxidase activity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, and whole plants. Definition ''In vitro'' ( la, in glass; often not italicized in English usage) studies are conducted using components of an organism that have been isolated fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
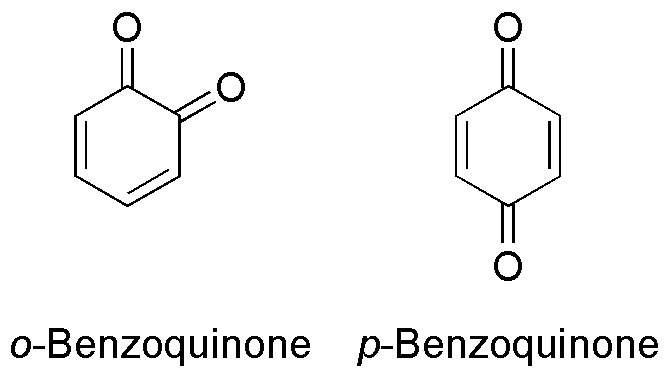
Benzoquinones
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of ''Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * I ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |