|
Antimicrobial Peptides
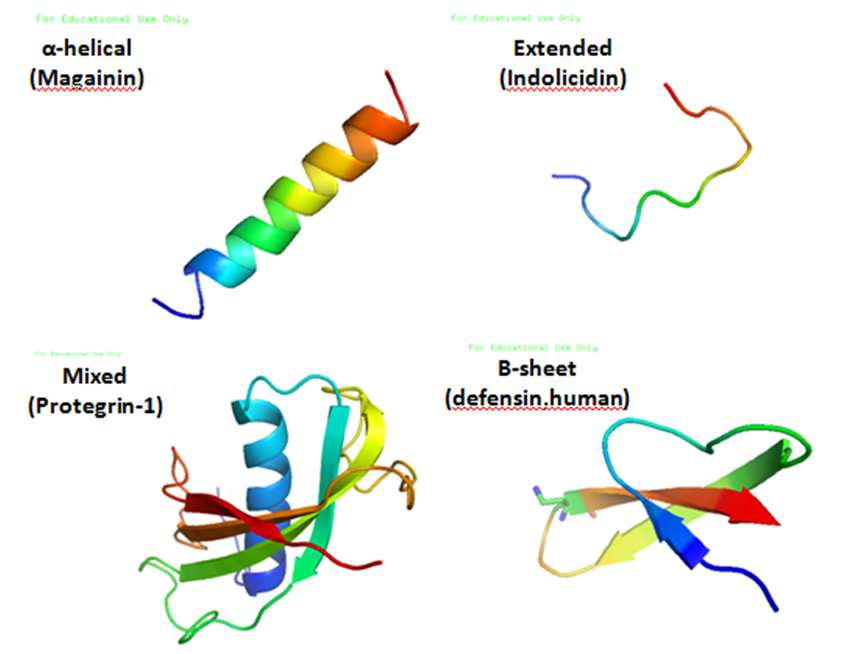
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Various AMPs
Various may refer to: * Various (band), an English dubstep/electronic music duo * Various artists, a term for a compilation album containing pieces by various musicians * Various authors, a book containing works by several writers * ''The Various'', a children's fantasy novel by Steve Augarde See also * Various & Gould, a Berlin-based artist duo * ''Various Artists – Archives Vol. 4'', an album by Steve Vai * ''Various Failures'', a compilation album by American experimental rock band Swans * ''The Various Haunts of Men'', a novel by Susan Hill * ''Various Positions'', an album by Leonard Cohen ** Various Positions Tour * ''Various Positions'' (film), a 2002 film directed by Ori Kowarsky * Varius (other) Varius is a Latin word meaning "diverse", "different", "changeable", "various" or "variegated" and may refer to: * ''Varius'' (moth), a genus of moths belonging to the small family Nepticulidae * Varius Manx, a Polish pop group * XKO Varius, a we ... * [Baidu] |
Disulfide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tachyplesin
Tachyplesin I and Tachyplesin II Tachyplesin is an antimicrobial peptide isolated from the horseshoe crab Horseshoe crabs are marine and brackish water arthropods of the family Limulidae and the only living members of the order Xiphosura. Despite their name, they are not true crabs or crustaceans: they are Chelicerata, chelicerates, most closely rela .... It has a molecular weight of 2.36 kDa and the amino acid sequence KWCFRVCYRGICYRRCR. References Further reading * Antimicrobial peptides {{Antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protegrin
Protegrins are small peptides containing 16-18 amino acid residues. Protegrins were first discovered in porcine leukocytes and were found to have antimicrobial activity against bacteria, fungi, and some enveloped viruses. The amino acid composition of protegrins contains six positively charged arginine residues and four cysteine residues. Their secondary structure is classified as cysteine-rich β-sheet antimicrobial peptides, AMPs, that display limited sequence similarity to certain defensins and tachyplesins. In solution, the peptides fold to form an anti-parallel β-strand with the structure stabilized by two cysteine bridges formed among the four cysteine residues. Recent studies suggest that protegrins can bind to lipopolysaccharide, a property that may help them to insert into the membranes of gram-negative bacteria and permeabilize them. Structure There are five known porcine protegrins, PG-1 to PG-5. Three were identified biochemically and rest of them were deduced from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indolicidin
Indolicidin is an antimicrobial peptide isolated from neutrophil Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying in ... blood cells of cows. The mature peptide is just 13 amino acids, making it one of the smallest antimicrobial peptides known to be encoded as the primary product of the encoding antimicrobial peptide gene. Indolicidin is active against bacterial pathogens, but has also been shown to kill fungi and even HIV virus. References {{organic-chem-stub Peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Attacin
Attacin is a glycine-rich protein of about 20 kDa belonging to the group of antimicrobial peptides (AMP). It is active against Gram-negative bacteria. Attacin was first discovered in '' Hyalophora cecropia'', but is widely conserved in different insects from butterflies to fruit flies. See also *Diptericin Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly '' Phormia terranova''. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein incl ..., a structurally related antimicrobial peptide References {{Reflist Insect immunity Antimicrobial peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diptericin
Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly '' Phormia terranova''. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein includes a proline-rich domain with similarities to the AMPs drosocin, pyrrhocoricin, and abaecin, and a glycine-rich domain with similarity to attacin. Diptericin is an iconic readout of immune system activity in flies, used ubiquitously in studies of ''Drosophila'' immunity. Diptericin is named after the insect order Diptera. Structure and function Diptericins are found throughout Diptera, but are most extensively characterized in ''Drosophila'' fruit flies. The mature structures of diptericins are unknown, though previous efforts to synthesize Diptericin have suggested Diptericin in ''Protophormia terraenovae'' is one linear peptide. Yet ''Drosophila melanogaster's'' Diptericin B peptide is likely cleaved into two separate peptides. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosocin
Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly ''Drosophila melanogaster'', and later shown to be conserved throughout the genus ''Drosophila''. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly. The ''Drosocin'' gene encodes two peptides: its namesake Drosocin peptide and a second peptide called Buletin. Structure and function Drosocin is primarily active against Gram-negative bacteria. The peptide is proline-rich with proline-arginine repeats, as well a critical threonine residue. This threonine is ''O''-glycosylated, which is required for antimicrobial activity. This ''O''-glycosylation can be performed either by mono- or disaccharides, which have different activity spectra. Like the antimicrobial peptides pyrrhocoricin and abaecin, drosocin binds to bacterial DnaK, inhibiting cell machinery and replication. The action of these drosocin-like peptides is potentiated by the presence of pore-forming pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LL37
Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the ''CAMP'' gene encodes the peptide precursor CAP-18 (18 kDa), which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body. Cathelicidin peptides are dual-natured molecules called amphiphiles: one end of the molecule is attracted to water and repelled by fats and proteins, and the other end is attracted to fat and proteins and repelled by water. Members of this family react to pathogens by disintegrating, damaging, or puncturing cell membranes. Cathelicidins thus serve a critical role in mammalian innate immune defense against invasive bacterial infection. The cathelicidin family of peptides are classified as antimicrobial peptides (AMPs). The AMP family also includes the defensins. Whilst the defensin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dermaseptin
Dermaseptins are a family of peptides isolated from skin of the frog genus '' Phyllomedusa''. The sequence of the dermaseptins varies greatly but due to the presence of lysine residues all are cationic and most have the potential to form amphipathic helices in water or when integrated with the lipid bilayer of the bacterial membrane. Clear separation of two lobes of positive and negative intramolecular electrostatic potential is thought to be important in cytotoxic activity. Dermaseptins are typically 27-34 amino acid residues in length and were the first vertebrate peptides demonstrated as having a lethal effect on the filamentous fungi implicated in severe opportunistic infections accompanying immunodeficiency syndrome and immunosuppressive drug therapy. Dermaseptin use in a novel drug delivery system has been proposed. The system is based on the affinity of dermaseptins for the plasma membrane of human erythrocytes. After transient loading of the cells with the non-toxic de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magainin
The magainins are a class of antimicrobial peptides found in the African clawed frog (''Xenopus laevis''). The peptides are cationic, generally lack a stable conformation in water but form amphipathic α-helix in membranes; their mechanism against micro-organisms is unclear but they disrupt the cell membranes of a broad spectrum of bacteria, protozoa, and fungi. They were independently discovered at around the same time by the labs of Michael Zasloff at the NIH and Dudley H. Williams at the University of Cambridge. They were named by Zasloff, after the Hebrew word for "shield," מגן māgēn (Ashkenazi pronunciation: magain). Zasloff helped found a company, Magainin Pharmaceuticals (subsequently called Genaera) to develop magainins into drugs. One candidate was an analog of magainin called pexiganan (MSI-78) that the company developed as a topical agent for infected diabetic foot ulcer Diabetic foot ulcer is a major complication of diabetes mellitus, and probably the major co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melittin
Melittin is the main component (40–60% of the dry weight) and the major pain producing substance of honeybee (''Apis mellifera'') venom. Melittin is a basic peptide consisting of 26 amino acids. Function The principal function of melittin as a component of bee venom is to cause pain and destruction of tissue of intruders that threaten a beehive. However, in honey bees, melittin is not only expressed in the venom gland, but also in other tissues when infected with pathogens. The two venom molecules, melittin and secapin, that are over-expressed in honey bees infected with various pathogens, possibly indicate a role for melittin in the immune response of bees to infectious diseases. Structure Melittin is a small peptide with no disulfide bridge; the ''N''-terminal part of the molecule is predominantly hydrophobic and the ''C''-terminal part is hydrophilic and strongly basic. In water, it forms a tetramer but it also can spontaneously integrate itself into cell membran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |