|
Anson Equation
In electrochemistry, the Anson equation defines the charge-time dependence for linear diffusion control in chronocoulometry.Chronoamperometry/chronocoulometry - Data Analysis https://www.basinc.com/manuals/EC_epsilon/Techniques/ChronoI/ca_analysis The Anson equation is written as: :Q = nFACD^\pi^t^ where, :Q = charge in coulombs :n = number of electrons (to reduce/oxidize one molecule of analyte) :F = Faraday constant, 96485 C/mol :A = area of the (planar) electrode in cm2 :C = concentration in mol/cm3; :D = diffusion coefficient in cm2/s :t = time in s. This is related to the Cottrell equation via integration with respect to time (t), and similarly implies that the electrode is planar. See also * Voltammetry * Electroanalytical methods * Limiting current * Cottrell equation References Electrochemical equations {{Electrochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
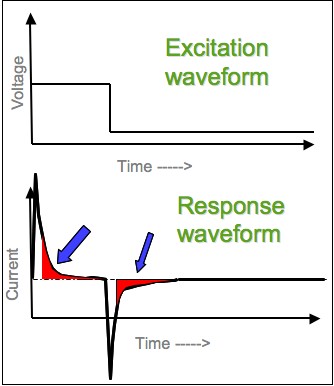
Chronoamperometry
Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode (caused by the potential step) is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells this decay is much slower than the charging decay-cells with n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coulomb
The coulomb (symbol: C) is the unit of electric charge in the International System of Units (SI). In the present version of the SI it is equal to the electric charge delivered by a 1 ampere constant current in 1 second and to elementary charges, , (about ). Name and history By 1878, the British Association for the Advancement of Science had defined the volt, ohm, and farad, but not the coulomb. In 1881, the International Electrical Congress, now the International Electrotechnical Commission (IEC), approved the volt as the unit for electromotive force, the ampere as the unit for electric current, and the coulomb as the unit of electric charge. At that time, the volt was defined as the potential difference .e., what is nowadays called the "voltage (difference)"across a conductor when a current of one ampere dissipates one watt of power. The coulomb (later "absolute coulomb" or "abcoulomb" for disambiguation) was part of the EMU system of units. The "international coul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faraday Constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of SI base units, which took effect on 20 May 2019, the Faraday constant has the exactly defined value given by the product of the elementary charge ''e'' and Avogadro constant ''N''A: : : :. Derivation The Faraday constant can be thought of as the conversion factor between the mole (used in chemistry) and the coulomb (used in physics and in practical electrical measurements), and is therefore of particular use in electrochemistry. Because 1 mole contains exactly entities, and 1 coulomb contains exactly elementary charges, the Faraday constant is given by the quotient of these two quantities: :. One common use of the Faraday constant is in electrolysis calculations. One can divide the amount of charge (the current integrated over time) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fick's Law Of Diffusion
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation. A diffusion process that obeys Fick's laws is called normal or Fickian diffusion; otherwise, it is called anomalous diffusion or non-Fickian diffusion. History In 1855, physiologist Adolf Fick first reported* * his now well-known laws governing the transport of mass through diffusive means. Fick's work was inspired by the earlier experiments of Thomas Graham, which fell short of proposing the fundamental laws for which Fick would become famous. Fick's law is analogous to the relationships discovered at the same epoch by other eminent scientists: Darcy's law (hydraulic flow), Ohm's law (charge transport), and Fourier's Law (heat transport). Fick's experiments (modeled on Graham's) dealt with measuring the concentrations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltammetry
Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram which plots the current produced by the analyte versus the potential of the working electrode. Theory Voltammetry is the study of current as a function of applied potential. Voltammetric methods involve electrochemical cells, and investigate the reactions occurring at electrode/electrolyte interfaces. The reactivity of analytes in these half-cells is used to determine their concentration. It is considered a dynamic electrochemical method as the applied potential is varied over time and the corresponding changes in current are measured. Most experiments control the potential(volts) of an electrode in contact with the analyte while measuring the resulting current (amperes) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroanalytical Method
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry (the difference in electrode potentials is measured), amperometry (electric current is the analytical signal), coulometry (charge passed during a certain time is recorded), and voltammetry (the cell's current is measured while actively altering the cell's potential). Potentiometry Potentiometry passively measures the potential of a solution between two electrodes, affecting the solution very little in the process. One electrode is called the reference electrode and has a constant potential, while the other one is an indicator electrode whose potential changes with the sample's composition. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limiting Current
The faradaic current is the current generated by the reduction or oxidation of some chemical substance at an electrode. The net faradaic current is the algebraic sum of all the faradaic currents flowing through an indicator electrode or working electrode. Limiting current The limiting current in electrochemistry is the limiting value of a faradaic current that is approached as the rate of charge transfer to an electrode is increased. The limiting current can be approached, for example, by increasing the electric potential or decreasing the rate of mass transfer to the electrode. It is independent of the applied potential over a finite range, and is usually evaluated by subtracting the appropriate residual current from the measured total current. A limiting current can have the character of an adsorption, catalytic, diffusion, or kinetic current, and may include a migration current. Migration current The difference between the current that is actually obtained, at any particular va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cottrell Equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step function in time. It was derived by Frederick Gardner Cottrell in 1903. For a simple redox event, such as the ferrocene/ferrocenium couple, the current measured depends on the rate at which the analyte diffuses to the electrode. That is, the current is said to be "diffusion controlled." The Cottrell equation describes the case for an electrode that is planar but can also be derived for spherical, cylindrical, and rectangular geometries by using the corresponding Laplace operator and boundary conditions in conjunction with Fick's second law of diffusion.Bard, A. J.; Faulkner, L. R. “Electrochemical Methods. Fundamentals and Applications” 2nd Ed. Wiley, New York. 2001. : i = \frac where, : i = current, in units of A : n = numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
