|
Anaplerotic Reaction
Anaplerotic reactions, a term coined by Hans Kornberg and originating from the Greeἀνά 'up' anπληρόω 'to fill', are chemical reactions that form intermediates of a metabolic pathway. Examples of such are found in the citric acid cycle (TCA cycle). In normal function of this cycle for respiration, concentrations of TCA intermediates remain constant; however, many biosynthetic reactions also use these molecules as a substrate. Anaplerosis is the Mitochondrion#Pyruvate and the citric acid cycle, act of replenishing TCA cycle intermediates that have been extracted for biosynthesis (in what are called anaplerotic reactions). The TCA cycle is a hub of metabolism, with central importance in both energy production and biosynthesis. Therefore, it is crucial for the cell to regulate concentrations of TCA cycle metabolites in the mitochondria. Anaplerotic flux must balance cataplerotic flux in order to retain homeostasis of cellular metabolism. Reactions of anaplerotic metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketoglutarate
Ketoglutaric acid or oxoglutaric acid, or its conjugate base, the carboxylate ketoglutarate or oxoglutarate, may refer to the following chemical compounds: * α-Ketoglutaric acid, an intermediate in the citric acid cycle The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins ... * β-Ketoglutaric acid (acetonedicarboxylic acid or 3-oxoglutaric acid) {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle. Etymology The word 'malic' is derived from Latin ' mālum', meaning 'apple'. The related Latin word , meaning 'apple tree', is used as the name of the genus ''Malus'', which includes all apples and crabapples; and the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae. Biochemistry L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. File:L-Äpfelsäure.svg, L-Malic acid File:D-Äpfelsäure.svg, D-Malic acid Malate plays an important role in biochemistry. In the C4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
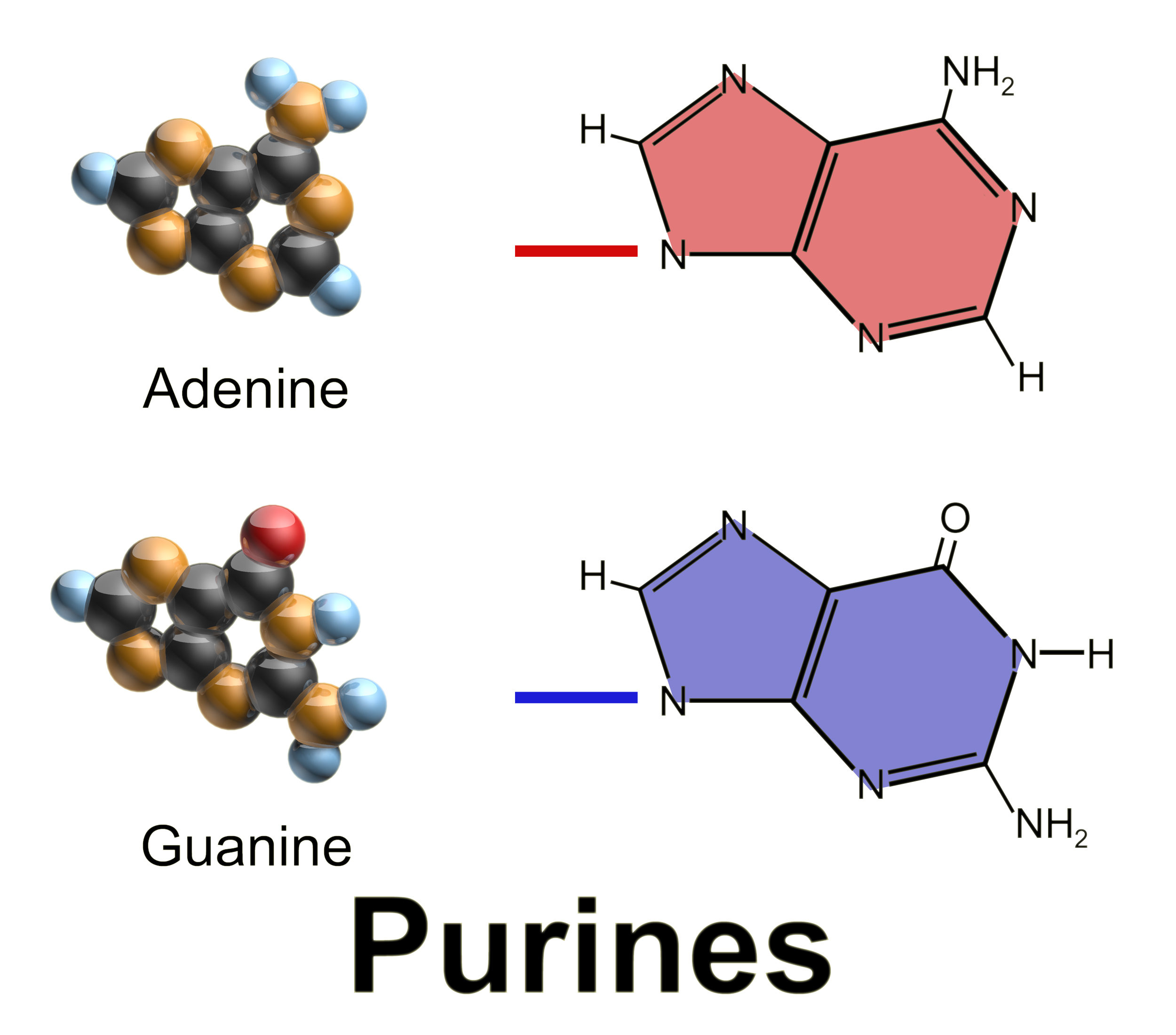
Purine Nucleotide Cycle
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, cauliflower, spi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenylosuccinate Lyase
Adenylosuccinate lyase (or adenylosuccinase) is an enzyme that in humans is encoded by the ADSL gene. Adenylosuccinate lyase converts adenylosuccinate to AMP and fumarate as part of the purine nucleotide cycle. ASL catalyzes two reactions in the purine biosynthetic pathway that makes AMP; ASL cleaves adenylosuccinate into AMP and fumarate, and cleaves SAICAR into AICAR and fumarate. Adenylosuccinate lyase is part of the β-elimination superfamily of enzymes and it proceeds through an E1cb reaction mechanism. The enzyme is a homotetramer with three domains in each monomer and four active sites per homotetramer. Point mutations in adenylosuccinate that cause lowered enzymatic activity cause clinical symptoms that mark the condition adenylosuccinate lyase deficiency. This protein may use the morpheein model of allosteric regulation. Function Adenylosuccinate lyase (ASL) is an enzyme that catalyzes two reactions in the ''de novo'' purine biosynthetic pathway. In both reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumarate
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297. The salts and esters are known as fumarates. Fumarate can also refer to the ion (in solution). Fumaric acid is the trans isomer of butenedioic acid, while maleic acid is the cis isomer. Biosynthesis and occurrence It is produced in eukaryotic organisms from succinate in complex 2 of the electron transport chain via the enzyme succinate dehydrogenase. It is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are ''trans'' (''E'') and in maleic acid they are ''cis'' (''Z''). Fumaric acid is found in fumitory (''Fumaria officinalis''), bolete mushrooms (specifically ''Boletus fomentarius var. pseudo-igniarius''), lichen, and Iceland moss. Fumarate is an intermediate in the citric acid cycle used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenylosuccinate
Adenylosuccinate is an intermediate in the interconversion of purine nucleotides inosine monophosphate (IMP) and adenosine monophosphate (AMP). The enzyme adenylosuccinate synthase carries out the reaction by the addition of aspartate to IMP and requires the input of energy from a phosphoanhydride bond in the form of guanosine triphosphate (GTP).Figures 20.4 and 20.7 in ''Textbook of Biochemistry'', with clinical correlations, Sixth Edition, Thomas M. Devlin, Ed., Wiley-Liss, Inc., New York, NY, 2006 GTP is used instead of adenosine triphosphate (ATP), so the reaction is not dependent on its products. See also * Adenylosuccinate lyase deficiency * Purine nucleotide cycle Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines a ... References Nucleotides {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triheptanoin
Triheptanoin, sold under the brand name Dojolvi, is a medication for the treatment of children and adults with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD). The most common adverse reactions include abdominal pain, diarrhea, vomiting, and nausea. Triheptanoin was approved for medical use in the United States in June 2020. Triheptanoin is a triglyceride that is composed of three seven-carbon (C7:0) fatty acids. These odd-carbon fatty acids are able to provide anaplerotic substrates for the TCA cycle. Triheptanoin is used clinically in humans to treat inherited metabolic diseases, such as pyruvate carboxylase deficiency and carnitine palmitoyltransferase II deficiency. It also appears to increase the efficacy of the ketogenic diet as a treatment for epilepsy. Since triheptanoin is composed of odd-carbon fatty acids, it can produce ketone bodies with five carbon atoms, as opposed to even-carbon fatty acids which are metabolized to ketone bodies with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonyl-CoA Mutase
Methylmalonyl-CoA mutase (, MCM), mitochondrial, also known as methylmalonyl-CoA isomerase, is a protein that in humans is encoded by the ''MUT'' gene. This vitamin B12-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in ''MUT'' gene may lead to various types of methylmalonic aciduria. MCM was first identified in rat liver and sheep kidney in 1955. In its latent form, it is 750 amino acids in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the N-terminus of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active holoenzyme form. Structure Gene The ''MUT'' gene lies on the chromosome location of 6p12.3 and consists of 13 exons, spanning over 35kb. Protein The mature enzyme is a homodimer with the N-terminal CoA binding domain and the C- terminal cobalam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acids
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsatura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinyl-CoA
Succinyl-coenzyme A, abbreviated as succinyl-CoA () or SucCoA, is a thioester of succinic acid and coenzyme A. Sources It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added. With B12 as an enzymatic cofactor, it is also synthesized from propionyl CoA, the odd-numbered fatty acid, which cannot undergo beta-oxidation. Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12-dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA. Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter the citric acid cycle. Fate It is converted into succinate through the hydrolytic release ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsaturati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |