|
Ammonia Solution
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests an alkali with composition , it is actually impossible to isolate samples of NH4OH. The ions and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions. Basicity of ammonia in water In aqueous solution, ammonia deprotonates a small fraction of the water to give ammonium and hydroxide according to the following equilibrium: : NH3 + H2O NH4+ + OH−. In a 1 M ammonia solution, about 0.42% of the ammonia is converted to ammonium, equivalent to pH = 11.63 because H4+ = 0.0042 M, H− = 0.0042 M, H3 = 0.9958 M, and pH = 14 + log10 H− = 11.62. The base ionization constant is : ''K''b = H4+OH−] / H3= 1.77. Sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Carbonate
Ammonium carbonate is a salt with the chemical formula (NH4)2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and is a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn, and produces a pungent smell when baked. Production Ammonium carbonate is produced by combining carbon dioxide and aqueous ammonia. About 80,000 tons/year were produced as of 1997. An orthorhombic monohydrate is known. It crystallizes in an ammonia solution exposed in a carbon dioxide-rich atmosphere. Decomposition Ammonium carbonate slowly decomposes at standard temperature and pressure through two pathways. Thus any initially pure sample of ammonium carbonate will soon become a mixture including various byproducts. Ammonium carbonate can spontaneously decompose into amm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Food Timeline
Lynne Olver (1958–2015) was a librarian and food historian, and the sole author of the Food Timeline website. Personal life Olver graduated from the University of Albany (SUNY). She was a librarian at the Morris County Library, New Jersey, and became its director in 2009. The Food Timeline In 1999, Olver created ''The Food Timeline'', a history website documenting culinary history, food history and recipes. The website has since become a major information source for culinary history. Almost all of the website's information comes from Lynne's personal library of over 2,000 books. Unlike many other food related websites, Olver gave citations to almost every statement on her site so that readers can verify her claims. Her research has been cited in peer-reviewed journals. Following her death, the site was given to her family, who chose to remove social media accounts associated with the Food Timeline, but kept the website running in a state of dormancy. As such, the websit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leavening Agent
In cooking, a leavening agent () or raising agent, also called a leaven () or leavener, is any one of a number of substances used in doughs and batters that cause a foaming action (gas bubbles) that lightens and softens the mixture. An alternative or supplement to leavening agents is mechanical action by which air is incorporated (i.e. kneading). Leavening agents can be biological or synthetic chemical compounds. The gas produced is often carbon dioxide, or occasionally hydrogen. When a dough or batter is mixed, the starch in the flour and the water in the dough form a matrix (often supported further by proteins like gluten or polysaccharides, such as pentosans or xanthan gum). The starch then gelatinizes and sets, leaving gas bubbles that remain. Biological leavening agents * ''Saccharomyces cerevisiae'' producing carbon dioxide found in: ** baker's yeast ** Beer barm (unpasteurised—live yeast) ** ginger beer ** kefir ** sourdough starter * ''Clostridium perfringens' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bicarbonate
Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia. Production Ammonium bicarbonate is produced by combining carbon dioxide and ammonia: :CO2 + NH3 + H2O → (NH4)HCO3 Since ammonium bicarbonate is thermally unstable, the reaction solution is kept cold, which allows the precipitation of the product as white solid. About 100,000 tons were produced in this way in 1997. Ammonia gas passed into a strong aqueous solution of the sesquicarbonate (a 2:1:1 mixture of (NH4)HCO3, (NH4)2CO3, and H2O) converts it into normal ammonium carbonate ((NH4)2CO3), which can be obtained in the crystalline condition from a solution prepared at about 30 °C. This compound on exposure to air gives off ammonia and reverts to ammonium bicarbonate. Salt of hartshorn Compositions c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
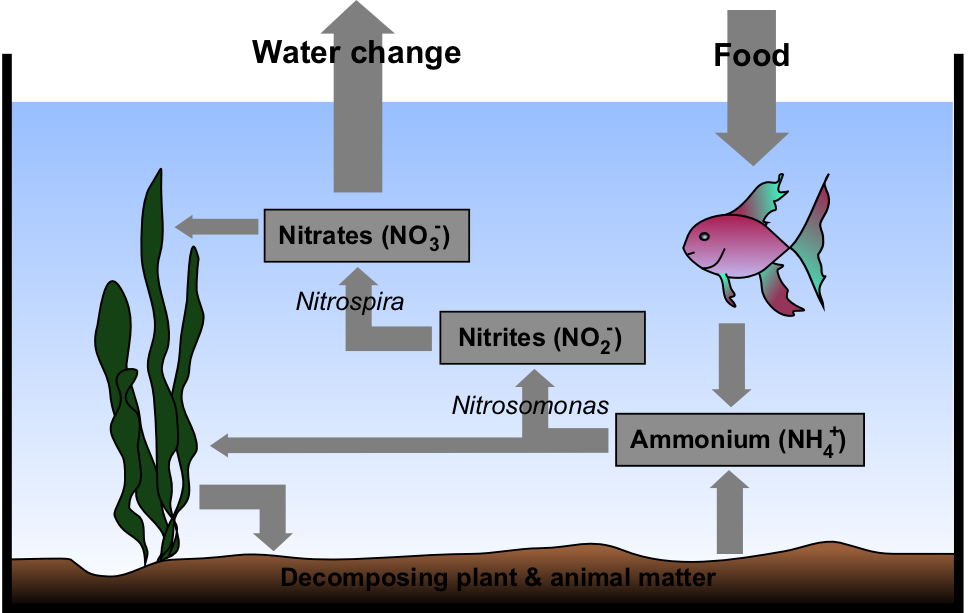
Fishless Cycling
Fishless cycling is a form of "maturing" an aquarium. In this process, ammonia is provided to allow beneficial bacteria to colonize. Fishless cycling can reduce the chance of fish loss resulting from insufficient populations of these bacteria. Process Fishless cycling takes place over a period of several weeks, during which the aquarist provides an ammonia source for the development of the nitrifying bacterial colony. Nitrifying bacteria in the aquarium grow on all surfaces, but particularly in areas of high water flow and high surface area such as the filter. Allowing ammonia to be converted to nitrite, and on to the less harmful nitrate, minimizes stress and injury to aquarium fish. The "nitrogen cycle" may take several weeks to complete, but may be quicker under certain conditions. Higher water temperatures, greater dissolved oxygen and bacterial seeding from an established tank may cut down the time required to less than a week. Advantages The most significant advantage o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fishkeeping
Fishkeeping is a popular hobby, practiced by aquarists, concerned with keeping fish in a home aquarium or garden pond. There is also a piscicultural fishkeeping industry, serving as a branch of agriculture. Origins of fishkeeping Fish have been raised as food in pools and ponds for thousands of years. Brightly colored or tame specimens of fish in these pools have sometimes been valued as pets rather than food. Many cultures, ancient and modern, have kept fish for both functional and decorative purposes. Ancient Sumerians kept wild-caught fish in ponds, before preparing them for meals. Depictions of the sacred fish of Oxyrhynchus kept in captivity in rectangular temple pools have been found in ancient Egyptian art. Similarly, Asia has experienced a long history of stocking rice paddies with freshwater fish suitable for eating, including various types of catfish and cyprinid. Selective breeding of carp into today's popular and completely domesticated koi and fancy gold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
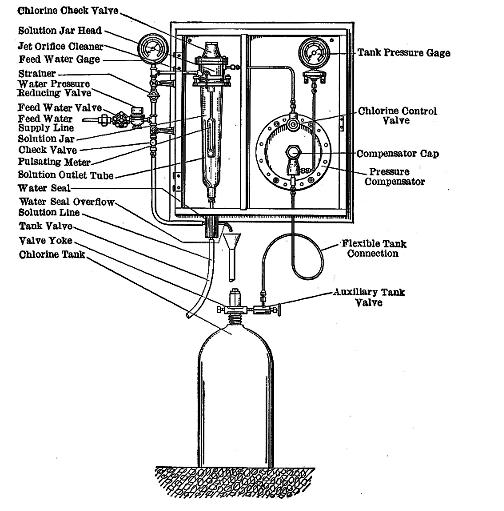
Water Chlorination
Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid. History In a paper published in 1894, it was formally proposed to add chlorine to water to render it "germ-free". Two other authorities endorsed this proposal and published it in many other papers in 1895. Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg, Germany. In 1897 the town of Maidstone, England was the first to have its entire water supply treated with chlorine. Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply caused a serious typhoid fever epidemic in Lincoln, England. Alexander Cruickshank Houston used chlorination of the water to stop the epidem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monochloramine
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of , but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured. Water treatment Chloramine is used as a disinfectant for water. It is less aggressive than chlorine and more stable against light than hypochlorites. Drinking water disinfection Chloramine is commonly used in low concentrations as a secondary disinfectant in municipal water distribution systems as an alternative to chlorination. This application is increasing. Chlorine (referred to in water treatment as free chlorine) is being displaced by chloramine—to be specific, monochloramine—which is much more stable and does not dissipate as rapidly as free ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Einstein Refrigerator
The Einstein–Szilard or Einstein refrigerator is an absorption refrigerator which has no moving parts, operates at constant pressure, and requires only a heat source to operate. It was jointly invented in 1926 by Albert Einstein and his former student Leó Szilárd, who patented it in the U.S. on November 11, 1930 (). The three working fluids in this design are water, ammonia, and butane. The Einstein refrigerator is a development of the original three-fluid patent by the Swedish inventors Baltzar von Platen and Carl Munters. History From 1926 until 1934 Einstein and Szilárd collaborated on ways to improve home refrigeration technology. The two were motivated by contemporary newspaper reports of a Berlin family who had been killed when a seal in their refrigerator failed and leaked toxic fumes into their home. Einstein and Szilárd proposed that a device without moving parts would eliminate the potential for seal failure, and explored practical applications for different r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Performance
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy (power) consumption and thus lower operating costs. The COP usually exceeds 1, especially in heat pumps, because, instead of just converting work to heat (which, if 100% efficient, would be a COP of 1), it pumps additional heat from a heat source to where the heat is required. Most air conditioners have a COP of 2.3 to 3.5. Less work is required to move heat than for conversion into heat, and because of this, heat pumps, air conditioners and refrigeration systems can have a coefficient of performance greater than one. However, this does not mean that they are more than 100% efficient, in other words, no heat engine can have a thermal efficiency of 100% or greater. For complete systems, COP calculations should include energy consumption o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |