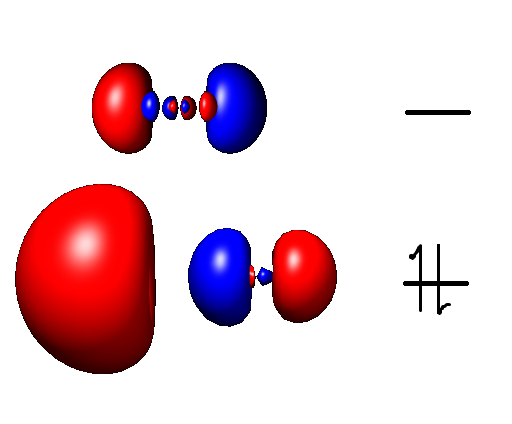|
Aluminium Monofluoride
Aluminium monofluoride, also known as fluoridoaluminium, is the chemical compound with the formula AlF. This elusive species is formed by the reaction between aluminium trifluoride and metallic aluminium at elevated temperatures but quickly reverts to the reactants when cooled. Clusters derived from related aluminium(I) halides can be stabilized using specialized ligands. This molecule has been detected in the interstellar medium, where molecules are so dilute that intermolecular collisions are unimportant. See also *Aluminium monobromide Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction i ... * Aluminium monochloride * Aluminium monoiodide References Aluminium(I) compounds Fluorides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monochloride
Aluminium monochloride, or chloridoaluminium is the metal halide with the formula AlCl. Aluminum monochloride as a molecule is thermodynamically stable at high temperature and low pressure only. This compound is produced as a step in the Alcan process to smelt aluminium from an aluminium-rich alloy. When the alloy is placed in a reactor that is heated to 1,300 °C and mixed with aluminium trichloride, a gas of aluminium monochloride is produced. : It then disproportionates into aluminium melt and aluminium trichloride upon cooling to 900 °C. This molecule has been detected in the interstellar medium, where molecules are so dilute that intermolecular collisions are unimportant. See also *Aluminium monofluoride *Aluminium monobromide Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium(I) Compounds
In chemistry, aluminium(I) refers to monovalent aluminium (+1 oxidation state) in both ionic and covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium. While late Group 13 elements such as thallium and indium prefer the +1 oxidation state, aluminium(I) is rare. Aluminium does not experience the inert pair effect, a phenomenon where valence s electrons are poorly shielded from nuclear charge due to the presence of filled d and f orbitals. As such, aluminium (III) (Al^3+) is the much more common oxidation state for aluminium. Aluminium(I) compounds are both prone to disproportionation and difficult to prepare. At standard conditions, they readily oxidize to the aluminium(III) form. Characteristics Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light. The geometry of compounds can be determined by analysis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monoiodide
Aluminium monoiodide is an aluminium(I) compound with the chemical formula AlI. It is unstable at room temperature due to dismutation: :6AlI -> + 4Al It forms a cyclic adduct Al4I4(NEt3)4 with triethylamine. See also *Aluminium monofluoride * Aluminium monochloride *Aluminium monobromide Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction i ... References Aluminium(I) compounds Iodides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monochloride
Aluminium monochloride, or chloridoaluminium is the metal halide with the formula AlCl. Aluminum monochloride as a molecule is thermodynamically stable at high temperature and low pressure only. This compound is produced as a step in the Alcan process to smelt aluminium from an aluminium-rich alloy. When the alloy is placed in a reactor that is heated to 1,300 °C and mixed with aluminium trichloride, a gas of aluminium monochloride is produced. : It then disproportionates into aluminium melt and aluminium trichloride upon cooling to 900 °C. This molecule has been detected in the interstellar medium, where molecules are so dilute that intermolecular collisions are unimportant. See also *Aluminium monofluoride *Aluminium monobromide Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monobromide
Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction is reversed at temperatures higher than 1000 °C. A more stable compound of aluminium and bromine is aluminium tribromide. See also *Aluminium monofluoride *Aluminium monochloride *Aluminium monoiodide Aluminium monoiodide is an aluminium(I) compound with the chemical formula AlI. It is unstable at room temperature due to dismutation: :6AlI -> + 4Al It forms a cyclic adduct Al4I4(NEt3)4 with triethylamine. See also *Aluminium monofluoride *A ... External links Aluminum monobromide NIST Standard Reference Data Program Aluminium(I) compounds Bromides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collision Theory
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the reactant hit each other with correct orientation, only a certain amount of collisions result in a perceptible or notable change; these successful changes are called successful collisions. The successful collisions must have enough energy, also known as activation energy, at the moment of impact to break the pre-existing bonds and form all new bonds. This results in the products of the reaction. Increasing the concentration of the reactant brings about more collisions and hence more successful collisions. Increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of collisions that have enough energy. Collision theory was proposed independently by Max Trautz in 1916 and William Lewis in 1918. When a catalyst is involved in the collision between the reactant molecules, less energy is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstellar Medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. The interstellar medium is composed of multiple phases distinguished by whether matter is ionic, atomic, or molecular, and the temperature and density of the matter. The interstellar medium is composed, primarily, of hydrogen, followed by helium with trace amounts of carbon, oxygen, and nitrogen. The thermal pressures of these phases are in rough equilibrium with one another. Magnetic fields and turbulent motions also provide pressure in the ISM, and are typically more important, dynamically, than the thermal pressure is. In the interstellar medium, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of formation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid, that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Formation of LiF from the elements releases one of the highest energy per mass of reactants, second only to that of BeO. Manufacturing LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride. Applications Precursor to LiPF6 for batteries Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make lithium hexafluorophosphate, an ingredient in lithium ion battery electrolyte. In molten salts Fluorine is produced by the electrolysis of molten potassium bifluoride. This electrolysis proceeds more efficiently when the electrolyte contains a few percent of LiF, possibl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Chemistry
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster'' may be used for ensembles with up to couple dozen atoms. Clusters with a definite number and type of atoms in a specific arrangement are often considered a specific chemical compound and are studied as such. For example, fullerene is a cluster of 60 carbon atoms arranged as the vertices of a truncated icosahedron, and decaborane is a cluster of 10 boron atoms forming an incomplete icosahedron, surrounded by 14 hydrogen atoms. The term is most commonly used for ensembles consisting of several atoms of the same element, or of a few different elements, bonded in a three-dimensional arrangement. Transition metals and main group elements form especially robust clusters. Indeed, in some contexts, the term may refer specifically to a met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-3D-balls.png)