|
Aluminide
An aluminide is a compound that has aluminium with more electropositive elements. Since aluminium is near the nonmetals on the periodic table, it can bond with metals differently from other metals. The properties of an aluminide are between those of a metal alloy and those of an ionic compound. Examples * Magnesium aluminide, MgAl * Titanium aluminide, TiAl * Iron aluminides, including Fe3Al and FeAl * Nickel aluminide Nickel aluminide typically refers to the one of the two most widely used compounds, Ni3Al or NiAl, however is generally any aluminide from the Ni-Al system. These alloys are widely used due to their corrosion resistance, low-density and easy product ..., Ni3Al See category for a list. * Intermetallics {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Aluminide
Iron aluminides are intermetallic compounds of iron and aluminium - they typically contain ~18% Al or more. Good oxide and sulfur resistance, with strength comparable to steel alloys, and low cost of materials have made these compounds of metallurgical interest - however low ductility and issues with hydrogen embrittlement are barriers to their processing and use in structural applications. Overview High corrosion resistance of Iron alloys containing more than 18% aluminium was first noted in the 1930s. Their tensile strength compares favorably with steels, whilst utilizing only common elements; however they have low ductility at room temperature, and strength drops of substantially over 600 °C. The alloys also have good sulfide and oxidation resistance, good wear resistance, and lower density than steels. Peak strength and hardness is reached at the Fe3Al stoichiometric region. Although Al gives corrosion resistance via an oxide film surface, reaction (with water) may also g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Aluminide
Titanium aluminide (chemical formula TiAl), commonly gamma titanium, is an intermetallic chemical compound. It is lightweight and resistant to oxidation and heat, but has low ductility. The density of γ-TiAl is about 4.0 g/cm3. It finds use in several applications including aircraft, jet engines, sporting equipment and automobiles. The development of TiAl based alloys began circa 1970. The alloys have been used in these applications only since about 2000. Titanium aluminide has three major intermetallic compounds: gamma titanium aluminide (gamma TiAl, γ-TiAl), alpha 2-Ti3Al and TiAl3. Among the three, gamma TiAl has received the most interest and applications. Applications of gamma-TiAl Gamma TiAl has excellent mechanical properties and oxidation and corrosion resistance at elevated temperatures (over 600°C), which makes it a possible replacement for traditional Ni based superalloy components in aircraft turbine engines. TiAl-based alloys have potential to increase the thru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
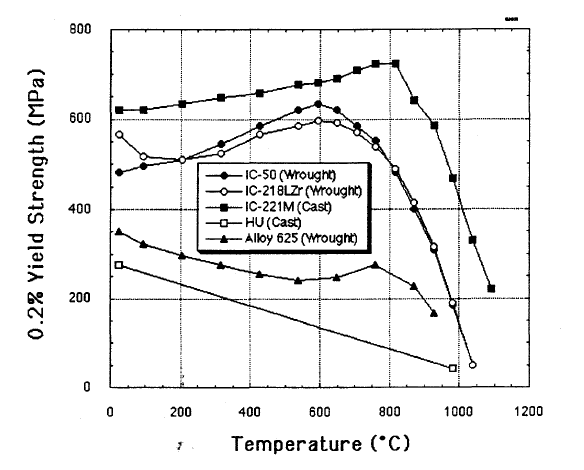
Nickel Aluminide
Nickel aluminide typically refers to the one of the two most widely used compounds, Ni3Al or NiAl, however is generally any aluminide from the Ni-Al system. These alloys are widely used due to their corrosion resistance, low-density and easy production. Ni3Al is of specific interest as the strengthening γ' phase precipitate in nickel-based superalloys allowing for high temperature strength up to 0.7-0.8 of its melting temperature. Meanwhile, NiAl displays excellent properties such as low-density (lower than that of Ni3Al), good thermal conductivity, oxidation resistance and high melting temperature. These properties, make it ideal for special high temperature applications like coatings on blades in gas turbines and jet engines. However, both these alloys do have the disadvantage of being quite brittle at room temperature while Ni3Al remains brittle at high temperatures as well. Although, it has been shown that Ni3Al can be made ductile when manufactured as a single crystal as oppos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Aluminide
Magnesium aluminide is an intermetallic compound of magnesium and aluminium. Common phases (molecular structures) include the beta phase (Mg2Al3) and the gamma phase (Mg17Al12), which both have cubic crystal structures. Magnesium aluminides are important constituents of 5XXX aluminium alloys (aluminium-magnesium) and magnesium-aluminium alloys, determining many of their engineering properties. Due to the advantage of low density and being strong, magnesium aluminide is important for aircraft engines. MgAl has also been investigated for use as a reactant to produce metal hydrides in hydrogen storage Hydrogen storage can be accomplished by several existing methods of holding hydrogen for later use. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand ... technology. Like many intermetallics, MgAl compounds often have unusual stoichiometries with large and complex unit cells. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
:Category:Aluminides
See aluminide An aluminide is a compound that has aluminium with more electropositive elements. Since aluminium is near the nonmetals on the periodic table, it can bond with metals differently from other metals. The properties of an aluminide are between those ... for definition. Aluminium compounds Intermetallics Anions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and Passivation (chemistry), forms a protective layer of Aluminium oxide, oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, Magnetism, non-magnetic and ductility, ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of Aluminum-26, 26Al is used in Radiometric dating, radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differently from those in metals. With some exceptions, those in nonmetals are fixed in place, resulting in nonmetals usually being poor conductors of heat and electricity and brittle or crumbly when solid. The electrons in metals are generally free moving and this is why metals are good conductors and most are easily flattened into sheets and drawn into wires. Nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds. Two nonmetals, hydrogen and helium, make up about 99% of ordinary matter in the observable universe by mass. Five nonmetallic elements, hydrogen, carbon, nitrogen, oxygen and silicon, largely make up the Earth's crust, atmosphere, oceans and biosphere. Most nonmetals have biological, technol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |