|
Alternative Periodic Tables
Alternative periodic tables are tabulations of chemical elements differing in their organization from the traditional depiction of the periodic system. Over a thousand have been devised, often for didactic reasons, as not all correlations between the chemical elements are effectively captured by the standard periodic table. Major alternative structures Left-step periodic table (Janet, 1928) Charles Janet's left-step periodic table is the most widely used alternative to the traditional depiction of the periodic system. It organizes elements according to an idealized orbital filling (instead of valence). For example, the elements Sc to Zn are shown as a 3d block implying orbital occupancy r4s2 3dx. (Although Cr and Cu are exceptions in the gas-phase, the idealized configurations are not too far away from the ground state, and the energy difference is small enough to be controlled by the chemical environment.) Compared to the common layout, the left-step table has these ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Giguère
Paul may refer to: *Paul (given name), a given name (includes a list of people with that name) *Paul (surname), a list of people People Christianity * Paul the Apostle (AD c.5–c.64/65), also known as Saul of Tarsus or Saint Paul, early Christian missionary and writer *Pope Paul (other), multiple Popes of the Roman Catholic Church *Saint Paul (other), multiple other people and locations named "Saint Paul" Roman and Byzantine empire *Lucius Aemilius Paullus Macedonicus (c. 229 BC – 160 BC), Roman general *Julius Paulus Prudentissimus (), Roman jurist *Paulus Catena (died 362), Roman notary *Paulus Alexandrinus (4th century), Hellenistic astrologer *Paul of Aegina or Paulus Aegineta (625–690), Greek surgeon Royals * Paul I of Russia (1754–1801), Tsar of Russia *Paul of Greece (1901–1964), King of Greece Other people * Paul the Deacon or Paulus Diaconus (c. 720 – c. 799), Italian Benedictine monk * Paul (father of Maurice), the father of Maurice, B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Block (periodic Table)
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, and f-block. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal quantum number: sharp (0), principal (1), diffuse (2), or fundamental (3). Succeeding notations proceed in alphabetical order, as g, h, etc., though elements that would belong in such blocks have not yet been found. Characteristics There is an ''approximate'' correspondence between this nomenclature of blocks, based on electronic configuration, and sets of elements based on chemical properties. The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block corresponds to the inner transitio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'', "gold-like" or "as gold"). It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role. Characteristics Zirconium is a lustrous, greyish-white, soft, ductile, malleable metal that is solid at room temperature, though it is hard and brittle at lesser purities. In powder form, zirconium is highl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
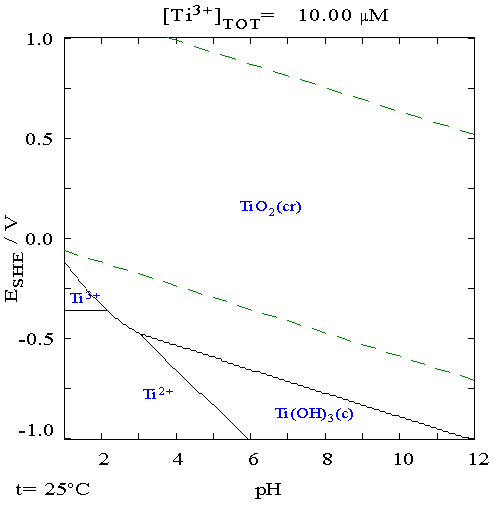
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and location ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 17 Element
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
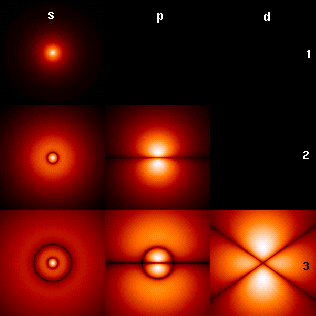
Magnetic Quantum Number
In atomic physics, the magnetic quantum number () is one of the four quantum numbers (the other three being the principal, azimuthal, and spin) which describe the unique quantum state of an electron. The magnetic quantum number distinguishes the orbitals available within a subshell, and is used to calculate the azimuthal component of the orientation of orbital in space. Electrons in a particular subshell (such as s, p, d, or f) are defined by values of (0, 1, 2, or 3). The value of can range from to , including zero. Thus the s, p, d, and f subshells contain 1, 3, 5, and 7 orbitals each, with values of within the ranges 0, ±1, ±2, ±3 respectively. Each of these orbitals can accommodate up to two electrons (with opposite spins), forming the basis of the periodic table. Derivation There is a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, \ell, m_\ell, and s specify the complete quantum state of a single electron in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Quantum Number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number, the magnetic quantum number, and the spin quantum number). It is also known as the orbital angular momentum quantum number, orbital quantum number or second quantum number, and is symbolized as ℓ (pronounced ''ell''). Derivation Connected with the energy states of the atom's electrons are four quantum numbers: ''n'', ''ℓ'', ''m''''ℓ'', and ''m''''s''. These specify the complete, unique quantum state of a single electron in an atom, and make up its wavefunction or ''orbital''. When solving to obtain the wave function, the Schrödinger equation reduces to three equations that lead to the first three quantum numbers. Therefore, the equations for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |