|
Alkyne Trimerisation
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles. Mechanism and stereochemistry Trimerisation of acetylene to benzene is highly exergonic, proceeding with a free energy change of 142 kcal/mol at room temperature. Kinetic barriers however prevent the reaction from proceeding smoothly. The breakthrough came in 1948, when Reppe and Schweckendiek reported their wartime results showing that nickel compounds are effective catalysts: : 3 RC2H -> C6R3H3 Since this discovery, many other cyclotrimerisations have been reported. Mechanism In terms of mechanism, the reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
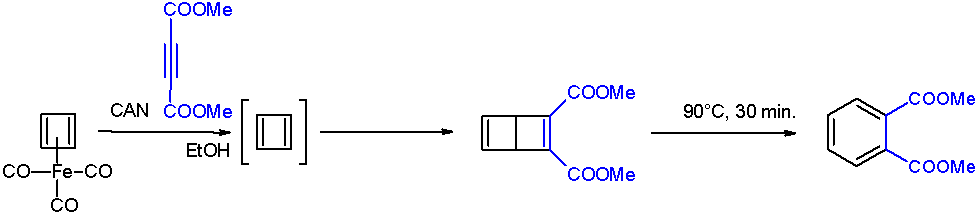
Benzocyclobutene
Benzocyclobutene (BCB) is a benzene ring fused to a cyclobutane ring. It has chemical formula . BCB is frequently used to create photosensitive polymers. BCB-based polymer dielectrics may be spun on or applied to various substrates for use in Micro Electro-Mechanical Systems (MEMS) and microelectronics processing. Applications include wafer bonding, optical interconnects, low-κ dielectrics, or even intracortical neural implants. Reactions Benzocyclobutene is a strained system which, upon heating to approximately 180 °C, causes the cyclobutene to undergo a conrotatory ring-opening reaction, forming ''o''-xylylene. Since this process destroys the aromaticity of the benzene ring, the reverse reaction is highly favored. ''o''-Xylylenes generated in this way have been used prolifically in cycloaddition reactions, which restore the aromaticity to the benzene ring, while forming a new annulated species. Uses The benzocyclobutene moiety has also appeared in a number of chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Tether
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place. Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocobalt Compound
Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl. Alkyl complexes Most fundamental are the cobalt complexes with only alkyl ligands. Examples include Co(4-norbornyl)4 and its cation. Alkylcobalt is represented by vitamin B12 and related enzymes. In methylcobalamin the ligand is a methyl group, which is electrophilic. in vitamin B12, the alkyl ligand is an adenosyl group. Related to vitamin B12 are cobalt porphyrins, dimethylglyoximates, and related complexes of Schiff base ligands. These synthetic compounds also form alkyl derivatives that undergo diverse reactions reminiscent of the biological processes. The weak cobalt(III)-carbon bond in vitamin B12 analogue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy. Unlike benzene, C6H6, cyclooctatetraene, C8H8, is not aromatic, although its dianion, ( cyclooctatetraenide), is. Its reactivity is characteristic of an ordinary polyene, i.e. it undergoes addition reactions. Benzene, by contrast, characteristically undergoes substitution reactions, not additions. History 1,3,5,7-Cyclooctatetraene was initially synthesized by Richard Willstätter in Munich in 1905 using pseudopelletierine as the starting material and the Hofmann elimination as the key transformation: : Willstätter noted that the compound did not exhibit the expected aromaticity. Between 1939 and 1943, chemists throug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enyne
In organic chemistry, an enyne is an organic compound containing a double bond (alkene) and a triple bond (alkyne). It is called a conjugated enyne when the double and triple bonds are conjugated. The term is a contraction of the terms alkene and alkyne. The simplest enyne is vinylacetylene. See also * Enyne metathesis *Enediyne *Polyyne In organic chemistry, a polyyne () is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural ... References Chemical nomenclature Alkene derivatives Alkyne derivatives Conjugated hydrocarbons {{organic-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexadiene
{{chemistry index ...
Cyclohexadiene may refer to: * 1,3-Cyclohexadiene, * 1,4-Cyclohexadiene, See also * Benzene or its theoretical isomer ''1,3,5-Cyclohexatriene'' * Cyclohexene Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n'' annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.png)