|
Acetylenic Diol Mechanism
In organic chemistry, the term acetylenic designates *A doubly unsaturated position (''sp''-hybridized) on a molecular framework, for instance in an alkyne such as acetylene; *An ethynyl fragment, HC\equivC–, or substituted homologue. See also * Allylic/Homoallylic * Benzylic * Propargylic/Homopropargylic * Vinylic In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contai ... Organic chemistry {{organic-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
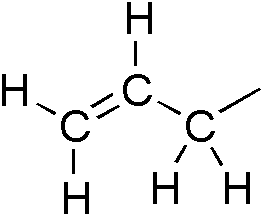
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°. Discovery Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolate potassium metal. By heating potassium carbonate with carbon at very high temperatures, he produced a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethynyl
In organic chemistry, the term ethynyl designates a functional group with a double bond with 2 carbon atoms both with sp hybridisation and a triple bond(1 sigma and 2 pi bond) . It is a species similar to acetylene (or in IUPAC ethyne ) with a less H atom thus joined to root chain. * An ethynyl group (HC≡C–), also designated as acetylenic group (from acetylene), and referred to in IUPAC chemical nomenclature as -yne suffix. Also sometimes designed as ''ethinyl'' in compounds (ethinylestradiol, ethisterone (ethinyltestosterone)). See main page alkynes. * The ethynyl radical (HC≡C·), the compound found in interstellar medium, and transiently on earth during chemical reactions. * The ethynyl carbanion Acetylide (HC≡C−) See also * Ethynylation * Propynyl ( H3C–C≡C–R, 1-propynyl group; or HC≡C–CH2–R, 2-propynyl group, Propargyl In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure . It is an alkyl group derived ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group. Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations The abbreviation "Bn" denotes benzyl. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propargylic
In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure . It is an alkyl group derived from propyne (). The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework next to an alkynyl group. The name comes from mix of ''propene'' and ''argentum'', which refers to the typical reaction of the terminal alkynes with silver salts. The term homopropargylic designates in the same manner * a saturated position on a molecular framework next to a propargylic group and thus two bonds from an alkyne moiety. * a 3-butynyl fragment, , or substituted homologue. See also * Alkenyl groups ** Allyl ** Vinyl group * Ethynyl * Propargyl chloride * Propargyl alcohol * Propargyl bromide * Propiolic acid Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decompo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinylic
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound containing that group, namely where R is any other group of atoms. An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as ''vinyl''. Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called '' divinyl benzene''.) Vinyl polymers Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers. Reactivity Vinyl derivatives are alkenes. If activat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |