|
Acetyl-CoA Synthase
Acetyl-CoA synthase (ACS), not to be confused with Acetyl-CoA synthetase or Acetate-CoA ligase (ADP forming), is a nickel-containing enzyme involved in the metabolic processes of cells. Together with Carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic organisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase. Chemistry In nature, there are six different pathways where CO is fixed. Of these, the Wood–Ljungdahl pathway is the predominant sink in anaerobic conditions. Acetyl-CoA Synthase (ACS) and carbon monoxide dehydrogenase (CODH) are integral enzymes in this one pathway and can perform diverse reactions in the carbon cycle as a result. Because of this, the exact activity of these molecules has come under inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA Synthetase
Acetyl-CoA synthetase (ACS) or Acetate—CoA ligase is an enzyme () involved in metabolism of acetate. It is in the ligase class of enzymes, meaning that it catalyzes the formation of a new chemical bond between two large molecules. Reaction The two molecules joined together that make up Acetyl CoA are acetate and coenzyme A (CoA). The complete reaction with all the substrates and products included is: : ATP + Acetate + CoA AMP + Pyrophosphate + Acetyl-CoA Once acetyl-CoA is formed it can be used in the TCA cycle in aerobic respiration to produce energy and electron carriers. This is an alternate method to starting the cycle, as the more common way is producing acetyl-CoA from pyruvate through the pyruvate dehydrogenase complex. The enzyme's activity takes place in the mitochondrial matrix so that the products are in the proper place to be used in the following metabolic steps. Acetyl Co-A can also be used in fatty acid synthesis, and a common function of the synthetase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ACS Autotrophic Growth And Acetate Synthesis
ACS or Acs may refer to: Organizations and societies * American Cancer Society, an American voluntary health organization dedicated to eliminating cancer * American Ceramic Society, an American professional organization * American Cheese Society, a professional organization of the American cheese industry * American Chemical Society, an American professional association * American College of Surgeons, a fellowship of American surgeons * American Colonization Society, an organization that helped in founding Liberia as a colony for freed slaves * American Constitution Society for Law and Policy, an organization of lawyers and law students in the US * American Cryonics Society, non-profit corporation that supports and promotes research and education into cryonics * American CueSports Alliance, a US-based pool league * Association of Caribbean States, an advisory, consultative body of Caribbean countries * Association of Cricket Statisticians and Historians, an association found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
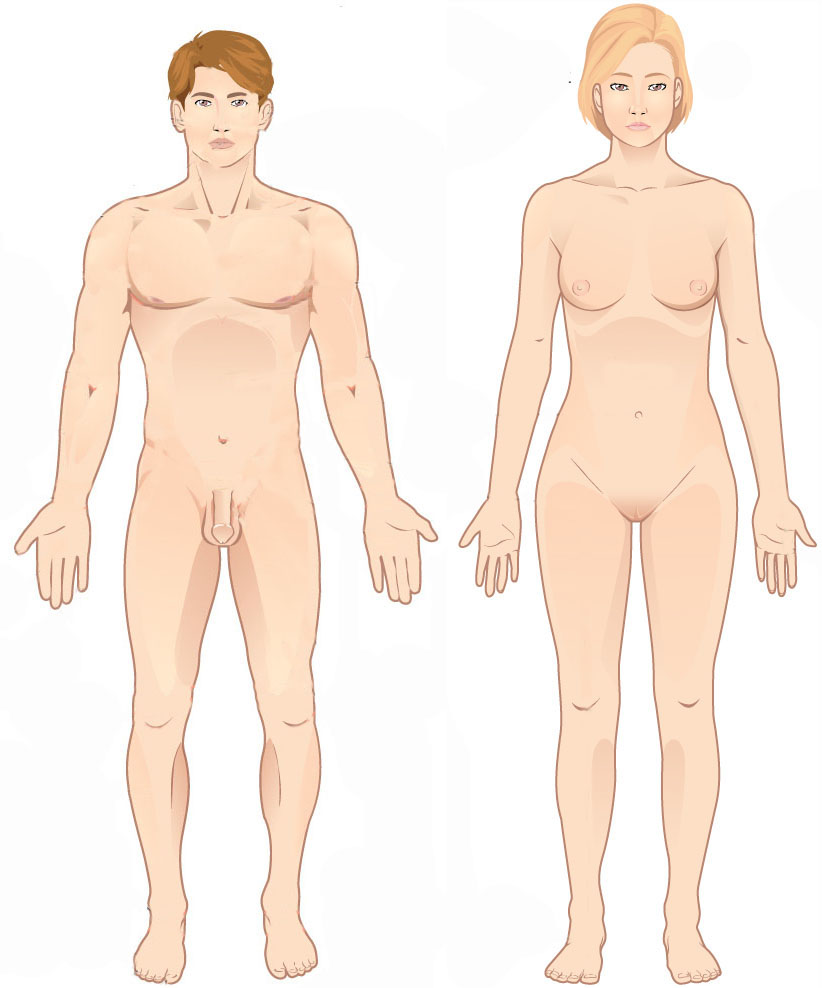
Proximal
Standard anatomical terms of location are used to unambiguously describe the anatomy of animals, including humans. The terms, typically derived from Latin or Greek roots, describe something in its standard anatomical position. This position provides a definition of what is at the front ("anterior"), behind ("posterior") and so on. As part of defining and describing terms, the body is described through the use of anatomical planes and anatomical axes. The meaning of terms that are used can change depending on whether an organism is bipedal or quadrupedal. Additionally, for some animals such as invertebrates, some terms may not have any meaning at all; for example, an animal that is radially symmetrical will have no anterior surface, but can still have a description that a part is close to the middle ("proximal") or further from the middle ("distal"). International organisations have determined vocabularies that are often used as standard vocabularies for subdisciplines of anatom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Square-planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corners. Examples Numerous compounds adopt this geometry, examples being especially numerous for transition metal complexes. The noble gas compound XeF4 adopts this structure as predicted by VSEPR theory. The geometry is prevalent for transition metal complexes with d8 configuration, which includes Rh(I), Ir(I), Pd(II), Pt(II), and Au(III). Notable examples include the anticancer drugs cisplatin tCl2(NH3)2and carboplatin. Many homogeneous catalysts are square planar in their resting state, such as Wilkinson's catalyst and Crabtree's catalyst. Other examples include Vaska's complex and Zeise's salt. Certain ligands (such as porphyrins) stabilize this geometry. Splitting of d-orbitals A general d-orbital splitting diagram for square planar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distal
Standard anatomical terms of location are used to unambiguously describe the anatomy of animals, including humans. The terms, typically derived from Latin or Greek roots, describe something in its standard anatomical position. This position provides a definition of what is at the front ("anterior"), behind ("posterior") and so on. As part of defining and describing terms, the body is described through the use of anatomical planes and anatomical axes. The meaning of terms that are used can change depending on whether an organism is bipedal or quadrupedal. Additionally, for some animals such as invertebrates, some terms may not have any meaning at all; for example, an animal that is radially symmetrical will have no anterior surface, but can still have a description that a part is close to the middle ("proximal") or further from the middle ("distal"). International organisations have determined vocabularies that are often used as standard vocabularies for subdisciplines of anatom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trinitrotoluene
Trinitrotoluene (), more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts. History TNT was first prepared in 1863 by German chemist Julius Wilbrand and originally used as a yellow dye. Its potential as an explosive was not recognized for three decades, mainly because it was too difficult to detonate because it was less sensitive than alternatives. Its explosive properties were first discovered in 1891 by another German chemist, Carl Häussermann. TNT can be safely poured when liquid into shell cases, and is so insensitive that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductase
A reductase is an enzyme that catalyzes a reduction reaction. Examples * 5α-Reductase * 5β-Reductase * Dihydrofolate reductase * HMG-CoA reductase * Methemoglobin reductase * Ribonucleotide reductase * Thioredoxin reductase * ''E. coli'' nitroreductase * Methylenetetrahydrofolate reductase See also * Oxidase * Oxidoreductase In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually u ... References Oxidoreductases {{Enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. Typical reducing agents undergo permanent chemical alteration through covalent or ionic reaction chemistry. This results in the complete and irreversible transfer of one or more electrons. In many chemical circumstances, however, the transfer of electronic charge to an electron acceptor may be only fractional, meaning an electron is not completely transferred, but results in an electron resonance between the donor and acceptor. This leads to the formation of charge transfer complexes in which the components largely retain their chemical identities. The electron donating power of a donor molecule is measured by its ionization potential which is the energy required to remove an electron from the highest occupied molecular orbital (HOMO). The overall energy balance (ΔE), i.e., energ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen. Nitrous oxide has significant medical uses, especially in surgery and dentistry, for its anaesthetic and pain-reducing effects. Its colloquial name, "laughing gas", coined by Humphry Davy, is due to the euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic. It is on the World Health Organization's List of Essential Medicines. It is also used as an oxidiser in rocket propellants, and in motor racing to increase the power output of engines. Nitrous oxide's atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of abo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |