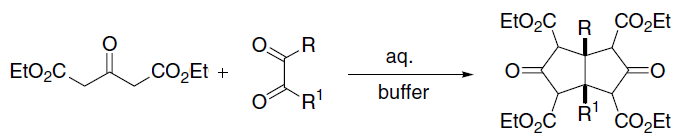
|
Acetonedicarboxylic Acid
Acetonedicarboxylic acid, 3-oxoglutaric acid or β-ketoglutaric acid is a simple dicarboxylic acid. Preparation Acetonedicarboxylic acid can also be prepared by decarboxylation of citric acid in fuming sulfuric acid: : Applications Acetonedicarboxylic acid and its esters such as dimethylacetonedicarboxylate are primarily used as building blocks in the synthesis of heterocyclic rings and in the Weiss–Cook reaction. Acetonedicarboxylic acid is well known to be used in the Robinson tropinone synthesis. The presence of β-ketoglutaric acid in human urine is diagnostic for the harmful gut flora such as ''Candida albicans ''Candida albicans'' is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usu ...''.Schmidt, Michael A, ''Tired of Being Tired: Overcoming Chronic Fatigue and Low Energy'' See also * alpha-K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most widely used dicarboxylic acid in the industry is adipic acid, which is a precursor in the production of nylon. Other examples of dicarboxylic acids include aspartic acid and glutamic acid, two amino acids in the human body. The name can be abbreviated to diacid. Linear saturated dicarboxylic acids The general formula is .Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. The PubChem links gives access to more information on the compounds, including other names, ids, toxicity and safety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. Its corresponding conjugate acid at pH 7.3 major species is known as tropiniumone. Synthesis The first synthesis of tropinone was by Richard Willstätter in 1901. It started from the seemingly related cycloheptanone, but required many steps to introduce the nitrogen bridge; the overall yield for the synthesis path is only 0.75%. Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine. Robinson's "double Mannich" reaction The 1917 synthesis by Robinson is considered a classic in total synthesis due to its simplicity and biomimetic approach. Tropinone is a bicyclic molecule, but the reactants used in its preparation are fairly simple: succinaldehyde, met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Candida Albicans
''Candida albicans'' is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usually a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions. It is one of the few species of the genus '' Candida'' that causes the human infection candidiasis, which results from an overgrowth of the fungus. Candidiasis is, for example, often observed in HIV-infected patients. ''C. albicans'' is the most common fungal species isolated from biofilms either formed on (permanent) implanted medical devices or on human tissue. ''C. albicans'', ''C. tropicalis'', ''C. parapsilosis'', and ''C. glabrata'' are together responsible for 50–90% of all cases of candidiasis in humans. A mortality rate of 40% has been reported for patients with systemic candidiasis due to ''C. albicans''. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarboxylic Acids
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most widely used dicarboxylic acid in the industry is adipic acid, which is a precursor in the production of nylon. Other examples of dicarboxylic acids include aspartic acid and glutamic acid, two amino acids in the human body. The name can be abbreviated to diacid. Linear saturated dicarboxylic acids The general formula is .Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. The PubChem links gives access to more information on the compounds, including other names, ids, toxicity and sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |