|
7-Methyl-DMT
7,''N'',''N''-trimethyltryptamine (7-methyl-DMT, 7-TMT), is a tryptamine derivative which acts as an agonist of 5-HT2 receptors. In animal tests, both 7-TMT and its 5-methoxy derivative 5-MeO-7-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT, but the larger 7-ethyl and 7-bromo derivatives of DMT did not produce psychedelic responses despite having higher 5-HT2 receptor affinity ''in vitro'' (cf. DOBU, DOAM). 7-TMT also weakly inhibits reuptake of serotonin but with little effect on dopamine or noradrenaline reuptake. See also * 2,N,N-TMT * 5,N,N-TMT * 7-Methyl-αET * 7-Chloro-AMT 7-Chloro-α-methyltryptamine (7-Cl-AMT) is a tryptamine derivative with stimulant effects, invented in the 1960s. It is a weak monoamine oxidase inhibitor but its pharmacology has not otherwise been studied by modern techniques, though several c ... References Serotonin receptor agonists Tryptamines Dimethylamino compounds {{nervous-system- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
7-Chloro-AMT
7-Chloro-α-methyltryptamine (7-Cl-AMT) is a tryptamine derivative with stimulant effects, invented in the 1960s. It is a weak monoamine oxidase inhibitor but its pharmacology has not otherwise been studied by modern techniques, though several closely related compounds are known to act as serotonin–dopamine releasing agents and agonists of the 5-HT2A receptor. See also * 5-Chloro-AMT * 5-Chloro-DMT * 5-Fluoro-AMT * 5-Fluoro-AET * 5-Fluoro-DMT * 6-Fluoro-AMT * 7-Methyl-DMT * 7-Methyl-AET * 7F-5-MeO-MET * O-4310 O-4310 (1-isopropyl-6-fluoro-psilocin) is a tryptamine derivative developed by Organix Inc which acts as a serotonin receptor agonist. It is claimed to have an EC50 of 5nM at 5-HT2A with 89% efficacy vs 5-HT, and 100x selectivity over 5-HT2C, ... References Designer drugs Psychedelic tryptamines Serotonin receptor agonists Chloroarenes {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
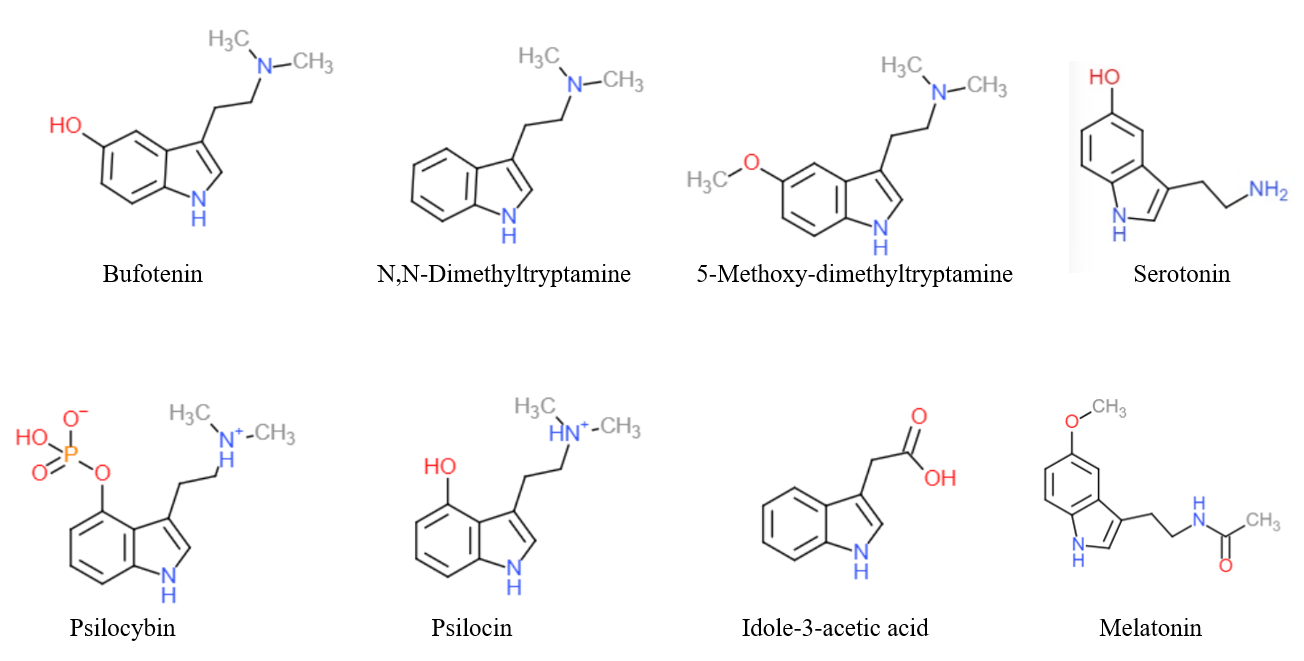
Tryptamines
Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms. Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "psilocybin mushrooms") and DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuasca brews. Many synthetic tryptamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Derivative
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is availa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2 Receptor
The 5-HT2 receptors are a subfamily of 5-HT receptors that bind the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT2 subfamily consists of three G protein-coupled receptors (GPCRs) which are coupled to Gq/G11 and mediate excitatory neurotransmission Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), ..., including 5-HT2A, 5-HT2B, and 5-HT2C. For more information, please see the respective main articles of the individual subtypes: * 5-HT2A receptor * 5-HT2B receptor * 5-HT2C receptor See also * 5-HT1 receptor * 5-HT3 receptor * 5-HT4 receptor * 5-HT5 receptor * 5-HT6 receptor * 5-HT7 receptor * 5-HT2 antagonists References {{Serotonergics Serotonin receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-7-TMT
5-Methoxy-7,N,N-trimethyltryptamine (5-MeO-7,N,N-TMT, 5-MeO-7-TMT), is a tryptamine derivative which acts as an agonist at the 5-HT2 serotonin receptors. In animal tests, both 7,N,N-TMT and 5-MeO-7,N,N-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT and 5-MeO-DMT, but compounds with larger 7-position substituents such as 7-ethyl-DMT and 7-bromo-DMT did not produce psychedelic-appropriate responding despite high 5-HT2 receptor binding affinity, suggesting these may be antagonists or weak partial agonists for the 5-HT2 receptors. The related compound 7-MeO-MiPT (cf. 5-MeO-MiPT 5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because ...) was also found to be inactive, suggesting that the 7-position has poor tolerance for bulky groups at this position, at l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychedelic Drug
Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness (known as psychedelic experiences or "trips").Pollan, Michael (2018). ''How to Change Your Mind: What the New Science of Psychedelics Teaches Us About Consciousness, Dying, Addiction, Depression, and Transcendence'' Sometimes, they are called classic hallucinogens, serotonergic hallucinogens, or serotonergic psychedelics, and the term ''psychedelics'' is used more broadly to include all hallucinogens; this article uses the narrower definition of ''psychedelics''. Psychedelics cause specific psychological, visual, and auditory changes, and often a substantially altered state of consciousness.Leary, Timothy; Metzner, Ralph (1964). ''The Psychedelic Experience: A Manual Based on The Tibetan Book of the Dead'' Psychedelic states are often compared to meditative, psychodynamic or transcendental types of alterations of mind. The "classical" psychedelics, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyltryptamine
''N'',''N''-Dimethyltryptamine (DMT or ''N'',''N''-DMT, SPL026) is a substituted tryptamine that occurs in many plants and animals, including human beings, and which is both a derivative and a structural analog of tryptamine. It is used as a psychedelic drug and prepared by various cultures for ritual purposes as an entheogen. DMT has a rapid onset, intense effects, and a relatively short duration of action. For those reasons, DMT was known as the "business trip" during the 1960s in the United States, as a user could access the full depth of a psychedelic experience in considerably less time than with other substances such as LSD or psilocybin mushrooms. DMT can be inhaled, ingested, or injected and its effects depend on the dose, as well as the mode of administration. When inhaled or injected, the effects last a short period of time: about five to 15 minutes. Effects can last three hours or more when orally ingested along with a monoamine oxidase inhibitor (MAOI), such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOBU
2,5-Dimethoxy-4-butylamphetamine (DOBU) is a lesser-known psychedelic drug and a substituted Amphetamine. DOBU was first synthesized by Alexander Shulgin. In his book '' PiHKAL (Phenethylamines i Have Known And Loved)'', only low dosages of 2–3 mg were tested, with the duration simply listed as "very long". DOBU produces paresthesia and difficulty sleeping, but with few other effects. Compared to shorter chain homologues such as DOM, DOET and DOPR which are all potent hallucinogens, DOBU has an even stronger 5-HT2 binding affinity but fails to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting it has low efficacy and is thus an antagonist or weak partial agonist at the 5-HT2A receptor. Isomers Alternative isomers of DOBU can also be produced, where the 4-(''n''-butyl) group of DOBU is replaced with any of the three other butyl isomers, the ''iso''-butyl, ''sec''-butyl and ''tert''-butyl compounds being called DOIB, DOSB and DO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DOAM
Dimethoxy-4-amylamphetamine (DOAM) is a lesser-known psychedelic drug and a substituted amphetamine. DOAM was first synthesized by Alexander Shulgin. In his book '' PiHKAL (Phenethylamines i Have Known And Loved)'', the minimum dosage is listed as 10 mg, and the duration is unknown. DOAM produces a bare threshold and tenseness. As the 4-alkyl chain length is increased from shorter homologues such as DOM, DOET and DOPR which are all potent hallucinogens, the 5-HT2 binding affinity increases, rising to a maximum with the 4-(''n''-hexyl) derivative before falling again with even longer chains, but compounds with chain length longer than ''n''-propyl, or with other bulky groups such as isopropyl, ''t''-butyl or γ-phenylpropyl at the 4- position, fail to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting these have low efficacy and are thus antagonists or partial agonists at the 5-HT2A receptor. See also * 2,5-Dimethoxy-4-Substitute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SSRI
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions. SSRIs increase the extracellular level of the neurotransmitter serotonin by limiting its reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine and dopamine transporters. SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations. Medical uses The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for anxiety disorders, such as social anxiety disorder, gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |