|
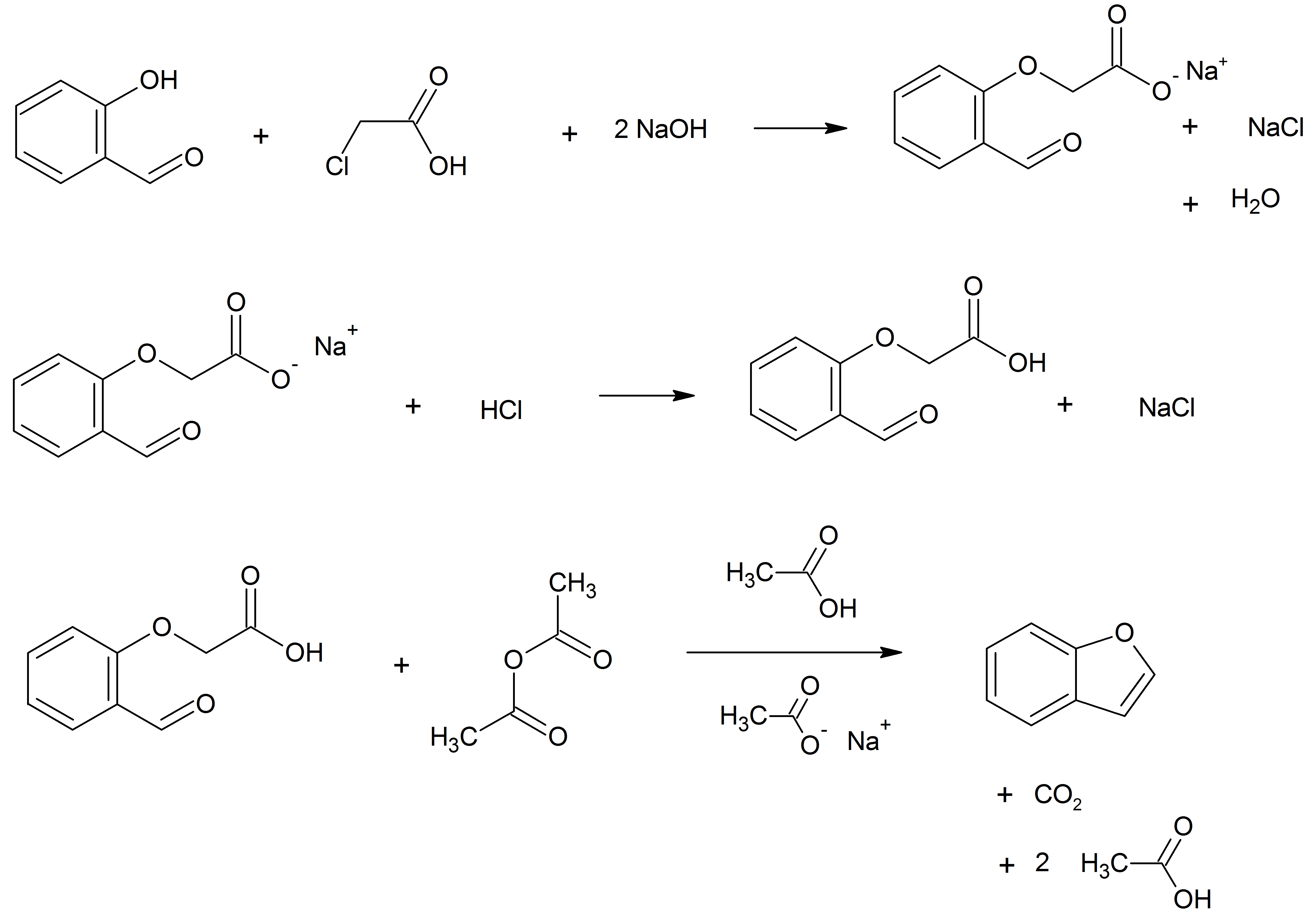
6-APB
6-APB (6-(2-aminopropyl)benzofuran) is an empathogenic psychoactive compound of the substituted benzofuran and substituted phenethylamine classes. 6-APB and other compounds are sometimes informally called "Benzofury" in newspaper reports. It is similar in structure to MDA, but differs in that the 3,4- methylenedioxyphenyl ring system has been replaced with a benzofuran ring. 6-APB is also the unsaturated benzofuran derivative of 6-APDB. It may appear as a tan grainy powder. While the drug never became particularly popular, it briefly entered the rave and underground clubbing scene in the UK before its sale and import were banned. It falls under the category of research chemicals, sometimes called "legal highs." Because 6-APB and other substituted benzofurans have not been explicitly outlawed in some countries, they are often technically legal, contributing to their popularity. Pharmacology Pharmacodynamics 6-APB is a serotonin–norepinephrine–dopamine reuptake inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6-APDB
6-(2-Aminopropyl)-2,3-dihydrobenzofuran (6-APDB, 4-Desoxy-MDA, EMA-3) is a stimulant and entactogen drug of the phenethylamine and amphetamine classes. It is an analogue of MDA where the heterocyclic 4-position oxygen from the 3,4-methylenedioxy ring has been replaced with a methylene bridge. 5-APDB (3-Desoxy-MDA) is an analogue of 6-APDB where the 3-position oxygen has been replaced with a methylene instead. 6-APDB, along with 5-APDB, was first synthesized by David E. Nichols in the early 1990s while investigating non-neurotoxic MDMA analogues. In animal studies, 6-APDB fully substitutes for MBDB and MMAI but not for amphetamine or LSD. ''In vitro'', 6-APDB has been shown to inhibit the reuptake of serotonin, dopamine, and norepinephrine with IC50 values of 322 nM, 1,997 nM, and 980 nM, respectively. These values are very similar to those of MDA, but with those for the catecholamines slightly lower in comparison, perhaps more similarly to MDMA. In contrast, 5-APDB is highly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2B Receptor
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the ''HTR2B'' gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Tissue distribution and function First discovered in the stomach of rats, 5-HT2B was challenging to characterize initially because of its structural similarity to the other 5-HT2 receptors, particularly 5-HT2C. The 5-HT2 receptors (of which the 5-HT2B receptor is a subtype) mediate many of the central and peripheral physiologic functions of serotonin. Cardiovascular effects include contraction of blood vessels and shape changes in platelets; central nervous system (CNS) effects include neuronal sensitization to tactile stimuli and mediation of some of the effects of hallucinogenic substituted amphetamines. The 5-HT2B receptor is expressed in several areas of the CNS, including the dorsal hypothalamus, frontal cortex, medial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychoactive Drug
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior. These substances may be used medically, recreationally or spiritually to a. Purposefully improve one’s perceived performance b. Alter one's consciousness (such as with entheogens for ritual, spiritual or shamanic purposes) or c. For research. Some categories of psychoactive drugs - which are believed, by some, to have therapeutic value - may be prescribed by some physicians and other healthcare practitioners. Examples of medication categories that may contain potentially beneficial psychoactive drugs include, but are not limited to: # Anesthetics # Analgesics # Anticonvulsants # Anti-Parkinson’s medications # Medications used to treat Neuropsychiatric Disorders a. Antidepressants b. Anxiolytics c. Antipsychotics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Benzofuran
The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached (at any position), and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name '' Benzofury''. List of substituted benzofurans The derivatives may be produced by substitutions at six locations of the benzofuran molecule, as well as saturation of the 2,3- double bond. The following table displays notable derivatives that have been reported: Legislation Substituted benzofurans saw widespread use as recreational drugs by being sold as research chemicals making the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Phenethylamine
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. The structural formula of any substituted phenethylamine contains a phenyl ring that is joined to an amino (NH) group via a two-carbon sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a specific group of atoms. Many substituted phenethylamines are psychoactive drugs which belong to a variety of different drug classes, including central nervous system stimulants (e.g., amphetamine), hallucinogens (e.g., dl- 2,5-dimethoxy-4-methylamphetamine DOM), entactogens (e.g., 3,4-methylenedioxyamphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofury (other)
{{disambig ...
Benzofury may refer to: * 5-APB * 5-MAPB * 6-APB * 6-MAPB 6-MAPB (1-(benzofuran-6-yl)-''N''-methylpropan-2-amine) is a psychedelic and entactogenic drug which is structurally related to 6-APB and MDMA 3,4-Methylenedioxymethamphetamine (MDMA), commonly seen in tablet form (ecstasy) and crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : Diels–Alder reaction yielding a substituted benzofuran, 450px * Cycloisomerization of alkyne ortho-substituted phenols: : Benzofurans via Cycloisomerization, 400px Related com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Transporter
Monoamine transporters (MATs) are protein structures that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. Three major classes of MATs (SERT, DAT, NET) are responsible for the reuptake of their associated amine neurotransmitters (serotonin, dopamine, norepinephrine). MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and posttranslational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
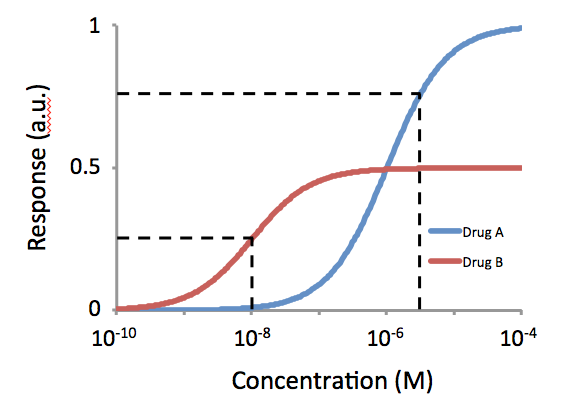
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Full Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |