|
5-Aza-7-deazapurine
5-Aza-7-deazapurine or imidazo,2-''a''1,3,5]triazine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of a s-triazine ring fused to an imidazole ring. It is an isostere and isomer of purine. However, in 5-aza-7-deazapurine, N-9 of five-membered ring does not bond with hydrogen. So 5-aza-7-deazapurine derivatives must have an exocyclic substituent with a double bond to bind a sugar residue. 5-Aza-7-deazapurine nucleosides may have an oxo, thioxo, or a imine group. Notable derivatives of this molecule include 5-aza-7-deazaguanine, which is a nucleobase of hachimoji DNA. See also * Base analog * Indolizine * Purine analogue Purine analogues are antimetabolites that mimic the structure of metabolic purines. Examples * Nucleobase analogues ** Thiopurines such as thioguanine are used to treat acute leukemias and remissions in acute granulocytic leukemias. ***Azathioprin ... References {{DEFAULTSORT:Aza-7-deazapurine, 5- Simple arom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-aza-7-deazaguanine
5-Aza-7-deazaguanine or 2-aminoimidazo ,2-a1,3,5]triazin-4(1H)-one is a 5-Aza-7-deazapurine base that is an isomer of guanine. It is used as a nucleobase of hachimoji DNA, in which it pairs with 6-Amino-5-nitropyridin-2-one 6-Amino-5-nitropyridin-2-one or 6-amino-5-nitro-2(1H)-pyridinone is a pyridine base. It is used as a nucleobase of hachimoji DNA, in which it pairs with 5-aza-7-deazaguanine 5-Aza-7-deazaguanine or 2-aminoimidazo,2-a1,3,5]triazin-4(1H)-one is .... References Nitrogen heterocycles Nucleobases {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers to either one of a pair of isomeric chemical compounds with molecular formula C3H4N2, having a five-membered ring consisting of three carbon atoms and two nitrogen atoms. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isostere
Classical Isosteres are molecules or ions with similar shape and often electronic properties. Many definitions are available. but the term is usually employed in the context of bioactivity and drug development. Such biologically-active compounds containing an isostere is called a bioisostere. This is frequently used in drug design: the bioisostere will still be recognized and accepted by the body, but its functions there will be altered as compared to the parent molecule. History and additional definitions Non-classical isosteres do not obey the above classifications, but they still produce similar biological effects in vivo. Non-classical isosteres may be made up of similar atoms, but their structures do not follow an easily definable set of rules. The isostere concept was formulated by Irving Langmuir in 1919, and later modified by Grimm. Hans Erlenmeyer extended the concept to biological systems in 1932.Mukesh Doble, Anil Kumar Kruthiventi, Vilas Gajanan. ''Biotransformations an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
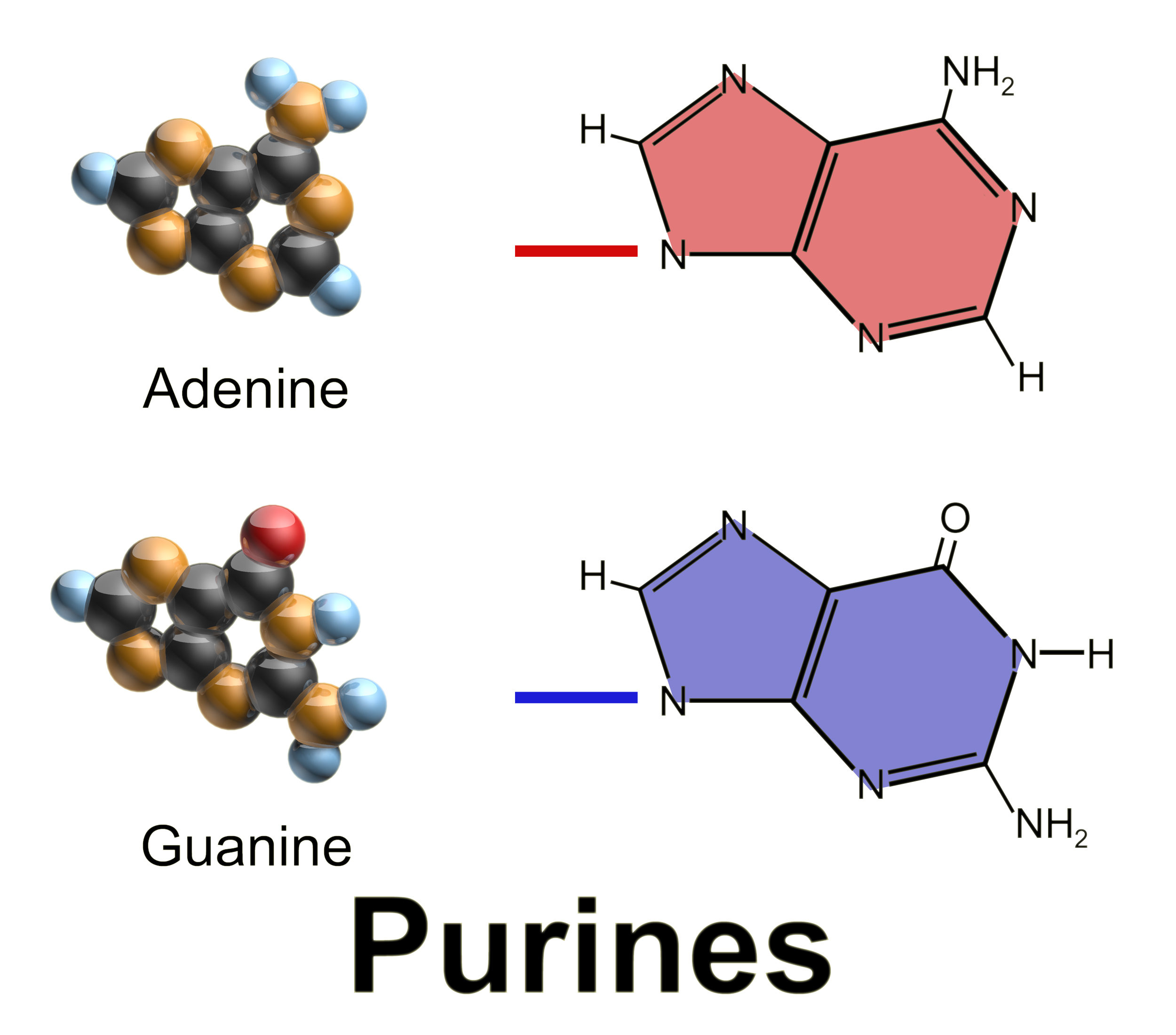
Purine
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and Black-eyed pea, black eye peas) and Spirulina (dietary supplement), spirulina. Examples of high-purine sources include: sweetbreads, Anchovies as food, anchovies, Sardines as food, sardines, liver, beef kidneys, Brain as food, brains, meat extracts (e.g., Oxo (food), Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleobase
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases—adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called ''primary'' or ''canonical''. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hachimoji DNA
Hachimoji DNA (from Japanese ''hachimoji'', "eight letters") is a synthetic nucleic acid analog that uses four synthetic nucleotides in addition to the four present in the natural nucleic acids, DNA and RNA. This leads to four allowed base pairs: two unnatural base pairs formed by the synthetic nucleobases in addition to the two normal pairs. Hachimoji bases have been demonstrated in both DNA and RNA analogs, using deoxyribose and ribose respectively as the backbone sugar. Benefits of such a nucleic acid system may include an enhanced ability to store data, as well as insights into what may be possible in the search for extraterrestrial life. The hachimoji DNA system produced one type of catalytic RNA (ribozyme or aptamer) ''in vitro''. Description Natural DNA is a molecule carrying the genetic instructions used in the growth, development, functioning, and reproduction of all known living organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids; alon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base Analog
Nucleic acid analogues are compounds which are Analog (chemistry), analogous (structurally similar) to naturally occurring RNA and DNA, used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides, which are composed of three parts: a phosphate backbone, a pentose sugar, either ribose or deoxyribose, and one of four nucleobases. An analogue may have any of these altered. Typically the analogue nucleobases confer, among other things, different base pairing and base stacking properties. Examples include universal bases, which can pair with all four canonical bases, and phosphate-sugar backbone analogues such as peptide nucleic acid, PNA, which affect the properties of the chain (PNA can even form a triple helix). Nucleic acid analogues are also called Xeno Nucleic Acid and represent one of the main pillars of xenobiology, the design of new-to-nature forms of life based on alternative biochemistries. Artificial nucleic acids include peptide nucleic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indolizine
Indolizine is an heterocyclic compound with the formula C8H7N). It is an uncommon isomer of indole with the nitrogen located at a ring fusion position. The saturated analogs are indolizidine, which are found in a variety of alkaloids such as swainsonine Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug. As a toxin in locoweed (likely its primary toxin) it also is a significant cause of economi .... References External links Chemical synthesis of indolizines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)