|
4-HO-DSBT
4-HO-DsBT (4-hydroxy-''N,N''-di-''sec''-butyltryptamine) is a tryptamine derivative which acts as a serotonin receptor agonist. It was first made by Alexander Shulgin and is mentioned in his book TiHKAL, but was never tested by him. However it has subsequently been tested ''in vitro'' and unlike the ''n''-butyl and isobutyl isomers which are much weaker, the ''s''-butyl derivative retains reasonable potency, with a similar 5-HT2A receptor affinity to MiPT but better selectivity over the 5-HT1A and 5-HT2B subtypes. See also * 4-HO-DiPT * 4-HO-DBT * 4-HO-McPeT * 4-HO-PiPT * 5-MeO-DBT * Dibutyltryptamine * N-t-Butyltryptamine * Robalzotan Robalzotan (NAD-299, AZD-7371) is a selective antagonist at the 5-HT1A receptor. It was shown to completely reverse the autoreceptor-mediated inhibition of serotonin release induced by the administration of selective serotonin reuptake inhibito ... References Tryptamines {{Pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-HO-McPeT
4-HO-McPeT (4-hydroxy-''N''-methyl-''N''-cyclopentyltryptamine) is a tryptamine derivative which has serotonergic effects. See also * 4-HO-DSBT * 4-HO-McPT * 4-HO-MPMI * 4-HO-pyr-T * 4-HO-DMT (Psilocin) * PiPT Propylisopropyltryptamine (PiPT) is a chemical in the tryptamine family, which reportedly produces psychedelic and hallucinogenic effects that resemble those of other related dialkyl tryptamine derivatives, although PiPT is reportedly relatively ... References Phenols Tryptamines {{pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-HO-PiPT
4-Hydroxy-''N''-propyl-''N''-isopropyltryptamine (4-HO-PiPT, Piprocin) is a substituted tryptamine derivative which is claimed to have psychedelic effects. It has been sold as a designer drug, first being identified in 2021 in British Columbia, Canada. See also * 4-HO-DPT * 4-HO-DiPT * 4-HO-DSBT * 4-HO-McPT * 4-HO-McPeT * 5-MeO-PiPT * Ebalzotan * Propylisopropyltryptamine Propylisopropyltryptamine (PiPT) is a chemical in the tryptamine family, which reportedly produces psychedelic and hallucinogenic effects that resemble those of other related dialkyl tryptamine derivatives, although PiPT is reportedly relatively ... References Tryptamines {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-MeO-DBT
5-MeO-DBT (5-Methoxy-N,N-dibutyltryptamine, 5-MeO-BET) is a rare substituted tryptamine derivative, which is thought to be a psychoactive substance and was identified in a designer drug sample by a forensic laboratory in Slovenia in March 2021, although only analytical studies have been conducted and no pharmacological data is available. It is nevertheless controlled under drug analogue legislation in a number of jurisdictions. See also * Dibutyltryptamine * 4-HO-DBT * 4-HO-DSBT * 5-MeO-DET 5-MeO-DET or 5-methoxy-''N,N''-diethyltryptamine is a hallucinogenic tryptamine. Pharmacology 5-MeO-DET inhibits serotonin reuptake with an IC50 value of 2.4 μM and activates 5-HT2A receptors with an EC50 value of 8.11 nM. Effects Low dosages ... * 5-MeO-EPT * 5-MeO-DPT References Tryptamines Methoxy compounds {{Pharm-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibutyltryptamine
''N,N''-Dibutyltryptamine (DBT) is a psychedelic drug belonging to the tryptamine family. It is found either as its crystalline hydrochloride salt or as an oily or crystalline base. DBT was first synthesized by the chemist Alexander Shulgin and reported in his book '' TiHKAL (Tryptamines i Have Known And Loved)''. Shulgin did not test DBT himself, but reports a human dosage of "1 mg/kg i.m." being active, but less so than DMT or DET. This suggests that an active dosage of DBT will be in the 100 mg range. This compound has been sold as a "research chemical" and has been confirmed to be an active hallucinogen although somewhat weaker than other similar tryptamine derivatives. It produces a head-twitch response in mice. There are four symmetrical isomers of DBT which can be made, or ten isomers in total if unsymmetrical substitution is used. Of these only the n-butyl analogue DBT is known to be active in humans; the isobutyl, sec-butyl, and tert-butyl isomers DIBT, DSBT and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
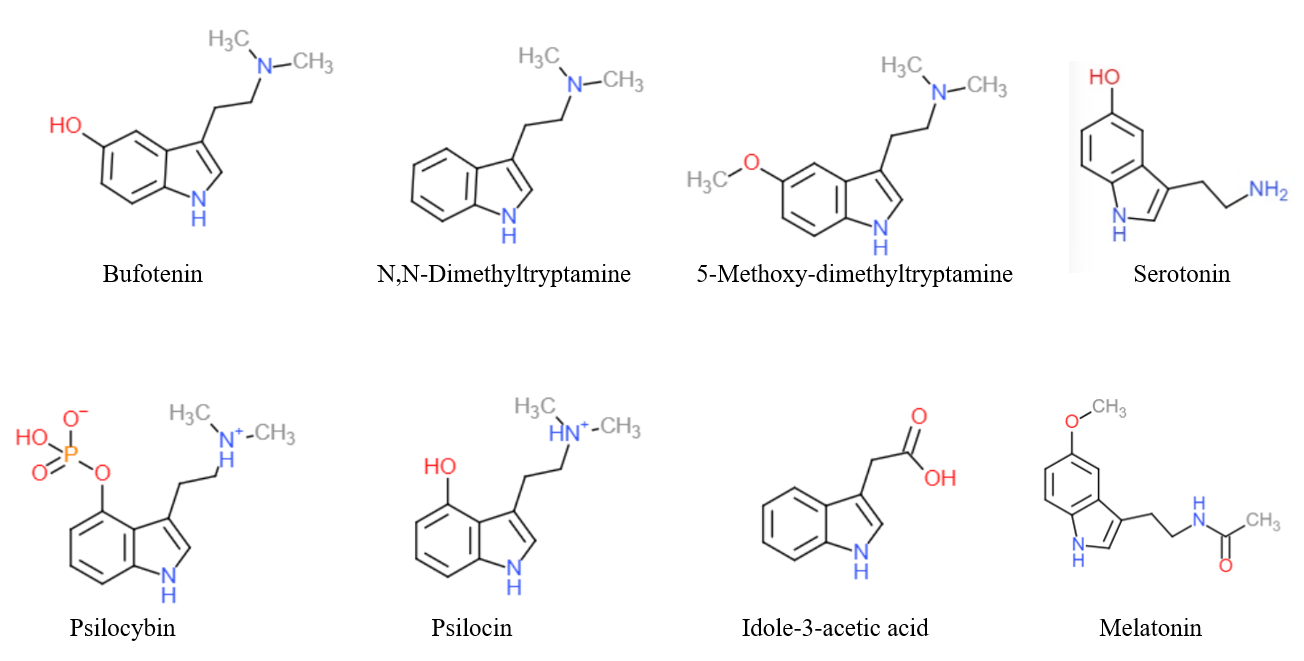
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. They are the target of a variety of pharmaceutical and recreational drugs, including many antidepressants, antipsychotics, anorectics, antiemetics, gast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Alexander Shulgin
Alexander Theodore "Sasha" Shulgin (June 17, 1925 – June 2, 2014) was an American medicinal chemist, biochemist, organic chemist, pharmacologist, psychopharmacologist, and author. He is credited with introducing 3,4-methylenedioxymethamphetamine (MDMA, commonly known as "ecstasy") to psychologists in the late 1970s for psychopharmaceutical use and for the discovery, synthesis and personal bioassay of over 230 psychoactive compounds for their psychedelic and entactogenic potential. In 1991 and 1997, he and his wife Ann Shulgin compiled the books '' PiHKAL'' and ''TiHKAL'' (standing for ''Phenethylamines'' and ''Tryptamines I Have Known And Loved''), from notebooks that extensively described their work and personal experiences with these two classes of psychoactive drugs. Shulgin performed seminal work into the descriptive synthesis of many of these compounds. Some of Shulgin's noteworthy discoveries include compounds of the 2C* family (such as 2C-B) and compounds of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MiPT
''N''-Methyl-''N''-isopropyltryptamine (MiPT) is a psychedelic tryptamine, closely related to DMT, DiPT and Miprocin. Chemistry MiPT base, unlike many other tryptamines in their freebase form, does not decompose rapidly in the presence of light or oxygen. In August 2019, Chadeayne et al. solved the crystal structure of MiPT fumarate. Its systematic name is -(1H-indol-3-yl)ethylmethyl)propan-2-ylazanium 3-carboxyprop-2-enoate. The salt consists of a protonated tryptammonium cation and a 3-carboxyacrylate (hydrogen fumarate) anion in the asymmetric unit. Dosage 10-25 mg is usually taken orally, with effects lasting 4–6 hours. Effects MiPT is said to emphasize psychedelic/entheogenic effects over sensory/hallucinogenic activity. Users report strong mental effects, but few perceptual alterations. Hyper sensitivity to sound as well Legality Sweden's public health agency suggested classifying MiPT as a hazardous substance, on May 15, 2019. MiPT is unscheduled in the Unit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1A Receptor
The serotonin 1A receptor (or 5-HT1A receptor) is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene. Distribution The 5-HT1A receptor is the most widespread of all the 5-HT receptors. In the central nervous system, 5-HT1A receptors exist in the cerebral cortex, hippocampus, septum, amygdala, and raphe nucleus in high densities, while low amounts also exist in the basal ganglia and thalamus. The 5-HT1A receptors in the raphe nucleus are largely somatodendritic autoreceptors, whereas those in other areas such as the hippocampus are postsynaptic receptors. Function Neuromodulation 5-HT1A recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2B Receptor
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the ''HTR2B'' gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Tissue distribution and function First discovered in the stomach of rats, 5-HT2B was challenging to characterize initially because of its structural similarity to the other 5-HT2 receptors, particularly 5-HT2C. The 5-HT2 receptors (of which the 5-HT2B receptor is a subtype) mediate many of the central and peripheral physiologic functions of serotonin. Cardiovascular effects include contraction of blood vessels and shape changes in platelets; central nervous system (CNS) effects include neuronal sensitization to tactile stimuli and mediation of some of the effects of hallucinogenic substituted amphetamines. The 5-HT2B receptor is expressed in several areas of the CNS, including the dorsal hypothalamus, frontal cortex, medial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |