|
4-Benzylpiperidine
4-Benzylpiperidine is a drug and research chemical used in scientific studies. It acts as a monoamine releasing agent with 20- to 48-fold selectivity for releasing dopamine versus serotonin. It is most efficacious as a releaser of norepinephrine, with an ec50 of 109/41.4/5246nM for DA/NE/5HT, respectively . It has a fast onset of action and a short duration. It also functions as a monoamine oxidase inhibitor Inhibitor or inhibition may refer to: In biology * Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity * Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotra ... (MAOI) with preference for MAO-A. Synthesis 4-Cyanopyridine can be reacted with toluene to give 4-benzylpyridine. Catalytic hydrogenation of the pyridine ring then completes the synthesis. See also * 2-Benzylpiperidine * Benzylpiperazine * Tetrahydroisoquinoline References Stimulants 4-Piperidinyl compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via insufflation (medicine), inhalation, drug injection, injection, smoking, ingestion, absorption (skin), absorption via a dermal patch, patch on the skin, suppository, or sublingual administration, dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to pharmacotherapy, treat, cure, preventive healthcare, prevent, or medical diagnosis, diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin. All monoamines are derived from aromatic amino acids like phenylalanine, tyrosine, and tryptophan by the action of aromatic amino acid decarboxylase enzymes. They are deactivated in the body by the enzymes known as monoamine oxidases which clip off the amine group. Monoaminergic systems, i.e., the networks of neurons that use monoamine neurotransmitters, are involved in the regulation of processes such as emotion, arousal, and certain types of memory. It has also been found that monoamine neurotransmitters play an important role in the secretion and production of neurotrophin-3 by astrocytes, a chemical which maintains neuron integrity and provides neurons with trophic support. Drugs used to increase or reduce the effect of monoamine neurotransmitters are u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Releasing Agent
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the synapse, release of a monoamine neurotransmitter from the synapse, presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds. Types of MRAs MRAS can be classified by the monoamines they mainly release, although these drugs lie on a spectrum. * Selective for one neurotransmitter ** Serotonin releasing agent (SRA) ** Norepinephrine releasing agent (NRA) ** Dopamine releasing agent (DRA) * Non-selective, releasing two or more neurotransmitters ** Norepinephrine–dopamine releasing agent (NDRA) ** Serotonin–norepinephrine releasing agent (SNRA) ** Serotonin–dopamine releasing agent (SDRA) ** Serotonin–norepinephrine–dopamine releasing agent (SNDR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic compound, organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor (chemistry), precursor chemical, L-DOPA, which is biosynthesis, synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopaminergic pathway, dopamine pathways, one of which plays a major role in the motivational component of reward system, reward-motivated behavior. The anticipa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular endothelium, gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
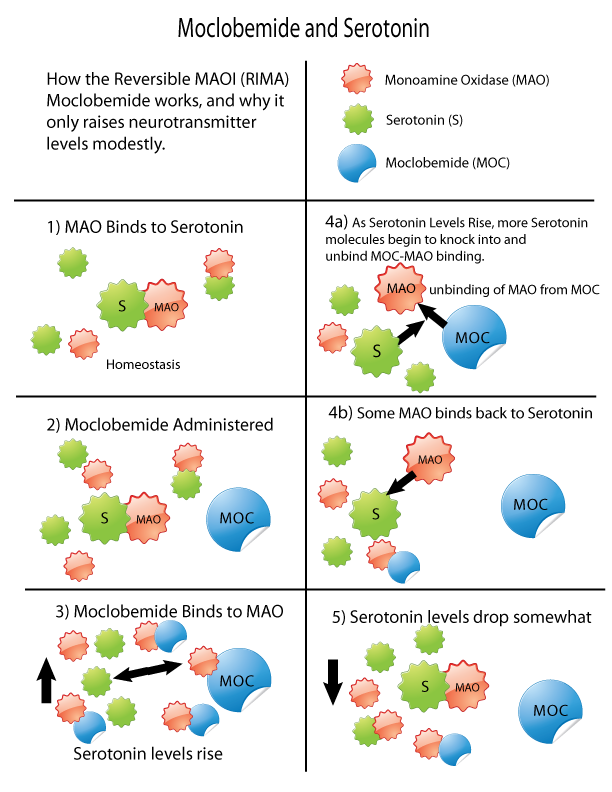
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase A
Monoamine oxidase A, also known as MAO-A, is an enzyme that in humans is encoded by the ''MAOA'' gene. This gene is one of two neighboring gene family members that encode mitochondrial enzymes which catalyze the oxidative deamination of amines, such as dopamine, norepinephrine, and serotonin. A mutation of this gene results in Brunner syndrome. This gene has also been associated with a variety of other psychiatric disorders, including antisocial behavior. Alternatively spliced transcript variants encoding multiple isoforms have been observed. Structures Gene Monoamine oxidase A, also known as MAO-A, is an enzyme that in humans is encoded by the ''MAOA'' gene. The promoter of ''MAOA'' contains conserved binding sites for Sp1, GATA2, and TBP. This gene is adjacent to a related gene (''MAOB'') on the opposite strand of the X chromosome. In humans, there is a 30-base repeat sequence repeated several different numbers of times in the promoter region of MAO-A. There are 2R (tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzylpiperazine
Benzylpiperazine (BZP) is a recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. Adverse effects have been reported following its use including acute psychosis, renal toxicity and seizures. Deaths from piperazine derivatives are extremely rare, but there has been at least one death apparently due to BZP alone. Its sale is banned in several countries, including Australia, Canada, New Zealand, the United States, the Republic of Ireland, the United Kingdom, Bulgaria, Romania and other parts of Europe. History Development history BZP was first synthesized by Burroughs Wellcome & Company in 1944. It is often claimed that it was originally synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals, but its synthesis is thought to pre-date their interest in piperazines as anthelmintics. Even so, the majority of the early work with the piperazines were investigations i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |