|
3-Mercaptopropionic Acid
3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulfide to acrylic acid. Reactions and uses It is competitive inhibitor of glutamate decarboxylase, and therefore acts as a convulsant. It has higher potency and faster onset of action compared to allylglycine. It is used to prepare hydrophilic gold nanoparticles, exploiting the affinity of gold for sulfur ligands. See also * Allylglycine * Thiolactic acid Thiolactic acid is the organosulfur compound with the formula HSCH2CO2H. The molecule contains both carboxylic acid and thiol functional groups. It is structurally related to lactic acid by the interchange of SH for OH. It is a colorless oil. T ... (2-mercaptopropionic acid) References Convulsants Glutamate decarboxylase inhibitors Thiols Propionic acids {{Organic-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bifunctional
In organic chemistry, when a single organic molecule has two different functional groups, it is called a bifunctional molecule . A bifunctional molecule has the properties of two different types of functional groups, such as an alcohol (), amide (), aldehyde (), nitrile () or carboxylic acid (). Many bifunctional molecules are used to produce medicines and catalysts, while others are used in condensation polymerization like polyester and polyamide. In organic molecules, functional groups are atoms or molecules that are responsible for the characteristic properties of that molecule, with the exceptions of double and triple bonds, which are also functional groups. See also * Functionality (chemistry) In chemistry, functionality is the presence of functional groups in a molecule. A monofunctional molecule possesses one functional group, a difunctional two, a trifunctional three, and so forth. In organic chemistry (and other fields of chemis ... References Further reading * Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylic Acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually. History The word "acrylic" was coined in 1843, for a chemical derivative of acrolein, an acrid-smelling oil derived from glycerol. Production Acrylic acid is produced by oxidation of propylene, which is a byproduct of the production of ethylene and gasoline: : 2 CH2=CHCH3 + 3 O2 → 2 CH2=CHCO2H + 2 H2O Historical methods Because acrylic acid and its esters have long been valued commercially, many other methods have been developed. Most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene (" Reppe chemistry"): : This method re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate Decarboxylase
Glutamate decarboxylase or glutamic acid decarboxylase (GAD) is an enzyme that catalyzes the decarboxylation of glutamate to gamma-aminobutyric acid (GABA) and carbon dioxide (). GAD uses pyridoxal-phosphate (PLP) as a cofactor. The reaction proceeds as follows: : In mammals, GAD exists in two isoforms with molecular weights of 67 and 65 kDa (GAD67 and GAD65), which are encoded by two different genes on different chromosomes (GAD1 and GAD2 genes, chromosomes 2 and 10 in humans, respectively). GAD67 and GAD65 are expressed in the brain where GABA is used as a neurotransmitter, and they are also expressed in the insulin-producing β-cells of the pancreas, in varying ratios depending upon the species. Together, these two enzymes maintain the major physiological supply of GABA in mammals, though it may also be synthesized from putrescine in the enteric nervous system, brain, and elsewhere by the actions of diamine oxidase and aldehyde dehydrogenase 1a1. Several truncated tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convulsant
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists (or inverse agonists) at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses (e.g. bupropion, tramadol, pethidine, dextropropoxyphene, clomipramine) but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onset Of Action
Onset of action is the duration of time it takes for a drug's effects to come to prominence upon administration. With oral administration, it typically ranges anywhere from 20 minutes to over an hour, depending on the drug in question. Other methods of ingestion such as smoking or injection can take as little as seconds to minutes to take effect. The determination of the onset of action, however, is not completely dependent upon route of administration. There are several other factors that determine the onset of action for a specific drug, including drug formulation, dosage, and the patient receiving the drug. Effect of Administration Route on the Onset of Action A drug's pharmacological effects can only occur once it has been fully solubilized and has entered the blood stream. For most drugs administered orally, the drug must be ingested, pass through the stomach, and into the small intestine, where the drug molecules enter the blood stream through the villi and microvilli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylglycine
Allylglycine is a glycine derivative. It is an inhibitor of glutamate decarboxylase. Inhibition of glutamate decarboxylase blocks GABA biosynthesis, leading to lower levels of the neurotransmitter. Allylglycine is known to induce seizures in animals studies, presumably due to this GDC-inhibiting activity. See also *3-Mercaptopropionic acid 3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulf ... References Alpha-Amino acids Convulsants Glutamate decarboxylase inhibitors Enzyme inhibitors Allyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
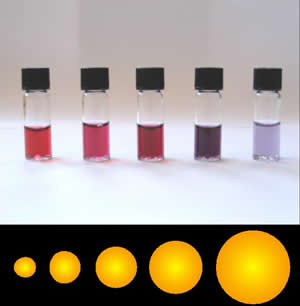
Gold Nanoparticle
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either wine-red coloured (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass colloidal gold was used in the 4th-century Lycurgus Cup, which changes color depending on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylglycine
Allylglycine is a glycine derivative. It is an inhibitor of glutamate decarboxylase. Inhibition of glutamate decarboxylase blocks GABA biosynthesis, leading to lower levels of the neurotransmitter. Allylglycine is known to induce seizures in animals studies, presumably due to this GDC-inhibiting activity. See also *3-Mercaptopropionic acid 3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulf ... References Alpha-Amino acids Convulsants Glutamate decarboxylase inhibitors Enzyme inhibitors Allyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiolactic Acid
Thiolactic acid is the organosulfur compound with the formula HSCH2CO2H. The molecule contains both carboxylic acid and thiol functional groups. It is structurally related to lactic acid by the interchange of SH for OH. It is a colorless oil. Thiolactic acid was once widely used in hair permanent waving formulations, but has been displaced by formulations based on thioglycolic acid. Instead of using the acid itself, its salts are used. It is now mainly used for depilation. See also * Hydroxybutyric acid * 3-Mercaptopropionic acid 3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulf ... References {{reflist Carboxylic acids Thiols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convulsants
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists (or inverse agonists) at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses (e.g. bupropion, tramadol, pethidine, dextropropoxyphene, clomipramine) but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |