|
1-ethynylcyclohexanol
1-Ethynylcyclohexanol (ECX) is an alkynyl alcohol derivative which is both a synthetic precursor to, and an active metabolite of the tranquilizer ethinamate, and has similar sedative, anticonvulsant and muscle relaxant effects. It has been sold as a designer drug, first being identified in the UK in March 2012. Preparation 1-Ethynylcyclohexanol can be prepared from cyclohexanone by the reacting it with sodium acetylide in liquid ammonia, followed by an acidic work-up. See also * 1,6-Dioxecane-2,7-dione * 2-Methyl-2-butanol * 2-Methyl-2-pentanol * 3-Methyl-3-pentanol * Clocental * Ethchlorvynol * Methylpentynol * Prenderol Prenderol (Diethylpropanediol) is a simple alkyl diol which has sedative, anticonvulsant and muscle relaxant effects. It is closely related in structure to meprobamate and numerous other alkyl alcohols and diols with generally comparable activity ... References Alcohols Muscle relaxants Ethynyl compounds {{musculoskeletal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-ethynylcyclohexanol Synthesis
1-Ethynylcyclohexanol (ECX) is an alkynyl alcohol derivative which is both a synthetic precursor to, and an active metabolite of the tranquilizer ethinamate, and has similar sedative, anticonvulsant and muscle relaxant effects. It has been sold as a designer drug, first being identified in the UK in March 2012. Preparation 1-Ethynylcyclohexanol can be prepared from cyclohexanone by the reacting it with sodium acetylide in liquid ammonia, followed by an acidic work-up. See also * 1,6-Dioxecane-2,7-dione * 2-Methyl-2-butanol * 2-Methyl-2-pentanol * 3-Methyl-3-pentanol * Clocental * Ethchlorvynol Ethchlorvynol is a GABA-ergic sedative and hypnotic/soporific medication first developed by Pfizer in the 1950s. In the United States it was sold by Abbott Laboratories under the trade name Placidyl. Placidyl was available in 200 mg, 500& ... * Methylpentynol * Prenderol References Alcohols Muscle relaxants Ethynyl compounds {{musculoskeletal-drug-st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethinamate
Ethinamate (Valamin, Valmid) is a short-acting carbamate-derivative sedative-hypnotic medication used to treat insomnia. Regular use leads to drug tolerance, and it is usually not effective for more than 7 days. Prolonged use can lead to dependency. Ethinamate has been replaced by other medicines (particularly benzodiazepines), and it is not available in the Netherlands, the United States or Canada. It is a schedule IV substance in the United States. Synthesis Ethinamate (1-ethynylcyclohexanone carbamate) is synthesized by combining acetylene with cyclohexanone to make 1-ethynylcyclohexanol, and then transforming this into a carbamate by the subsequent reaction with phosgene, and later with ammonia Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa .... Some lithium metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,6-Dioxecane-2,7-dione
1,6-Dioxecane-2,7-dione is a chemical compound classified as a lactone. It is formed as an impurity in the manufacture of polymer resins and biodegradable polyesters. It is the cyclic dimer of GHB and has been sold as a designer drug. See also * Aceburic acid * Ethyl acetoxy butanoate * gamma-Butyrolactone * gamma-Hydroxybutyraldehyde γ-Hydroxybutyraldehyde is the organic compound with the formula HOCH2CH2CH2CHO. It is a colorless liquid. The compound occurs in nature and is produced commercially. Occurrence It is a chemical intermediate in the biosynthesis of the neurotra ... References Lactones {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenderol
Prenderol (Diethylpropanediol) is a simple alkyl diol which has sedative, anticonvulsant and muscle relaxant effects. It is closely related in structure to meprobamate and numerous other alkyl alcohols and diols with generally comparable activity.Reyes Q., Aaurelio; Mascetti V., G.; Martinez J., Rolando. Synthesis of polyhydroxylated alcohols. ''Revista Latinoamericana de Quimica'' 1984; 15 (1): 29-30. ISSN: 0370-5943. See also * 1,4-Butanediol * 1,6-Dioxecane-2,7-dione * 1-Ethynylcyclohexanol * 2-Methyl-2-propyl-1,3-propanediol * 2-Methyl-2-butanol * 2-Methyl-2-pentanol * 3-Methyl-3-pentanol * 3-Hydroxybutanal * Ethchlorvynol * Phenaglycodol Phenaglycodol (brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran) is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties. It is relat ... References Alkanediols Muscle relaxants {{musculoskeletal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylpentynol
Methylpentynol (Methylparafynol, Dormison, Atemorin, Oblivon) is a tertiary hexynol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles. The drug was marketed again in the United States, Europe and elsewhere from 1956 well into the 1960s as a rapid-acting sedative. The drug was quickly overshadowed at that point by benzodiazepines and is no longer sold anywhere. Synthesis Methylpentynol is prepared by reaction of butanone (MEK) with sodium acetylide. This reaction must be done in anhydrous conditions and in an inert atmosphere. Applications As building block in the synthesis of: # Phthalofyne (1,2-Benzenedicarboxylic acid, mono(1-ethyl-1-methyl-2-propynyl) ester) 31-67-9# Anansiol (1-ethyl-1-methylprop-2-ynyl carbamate) 02-66-9# Bason ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethchlorvynol
Ethchlorvynol is a GABA-ergic sedative and hypnotic/soporific medication first developed by Pfizer in the 1950s. In the United States it was sold by Abbott Laboratories under the trade name Placidyl. Placidyl was available in 200 mg, 500 mg, and 750 mg strength gel filled capsules. While the 500 mg and 750 mg strength capsules were for use in reducing sleep latency, the 200 mg strength capsules were intended to be used to re-induce sleep in case of early awakening. Abbott discontinued production in 1999, due to it being replaced by the benzodiazepine family and its widespread abuse, after which Placidyl was available for about a year in the United States. Although, theoretically, ethchlorvynol could be manufactured for sale in the United States by another pharmaceutical company (subject to FDA approval of such manufacture), no pharmaceutical company has chosen to do so. Individuals with a valid prescription for the substance may legally transpor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clocental
Clocental (dolcental) is a carbamate-derived sedative hypnotic. It can be prepared by the ethynylation of cyclohexanone followed by reaction with allophanyl chloride. See also * 1-Ethynylcyclohexanol * Methylpentynol Methylpentynol (Methylparafynol, Dormison, Atemorin, Oblivon) is a tertiary hexynol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter f ... References {{GABAAR PAMs Ethynyl compounds Carbamates Hypnotics Sedatives GABAA receptor positive allosteric modulators ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
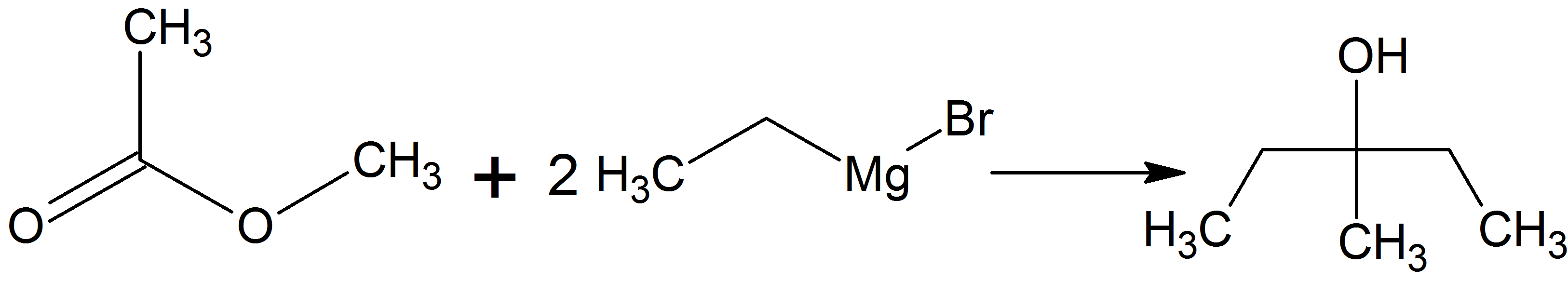
3-Methyl-3-pentanol
3-Methyl-3-pentanol (IUPAC name: 3-methylpentan-3-ol) is an organic chemical compound and a tertiary hexanol. It is used in the synthesis of the tranquilizer emylcamate, and has similar sedative and anticonvulsant actions itself. Synthesis It can be prepared by reacting ethylmagnesium bromide with methyl acetate in the so-called Grignard reaction using dried diethyl ether or tetrahydrofuran as solvent. It can be prepared also by reacting ethylmagnesium bromide with butanone Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in ... in the same conditions already mentioned. References Tertiary alcohols Hexanols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methyl-2-pentanol
2-Methyl-2-pentanol (IUPAC name: 2-methylpentan-2-ol) is an organic chemical compound. It can be added to a gas chromatograph to help distinguish between branched compounds, especially alcohols. Its presence in urine can be used to test for exposure to 2-methylpentane. As with many other short-chain alcohols, 2-methyl-2-pentanol can produce intoxication and sedative effects similar to those of ethanol Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ..., though it is more irritating to mucous membranes and generally more toxic to the body. See also * 2-Methyl-2-butanol * 3-Methyl-3-pentanol References Hexanols Tertiary alcohols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methyl-2-butanol
''tert''-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol. Historically TAA has been used an anesthetic and more recently used as a recreational drug. TAA is mostly a positive allosteric modulator for GABAA receptors in the same way as ethanol. The psychotropic effects of TAA and ethanol are similar, though distinct. Impact on coordination and balance are proportionately more prominent with TAA, which is significantly more potent by weight than ethanol. TAA is a colorless liquid with a burning flavor and an unpleasant odor similar to paraldehyde with a hint of camphor. TAA remains as a liquid at room temperature making it a useful alternative solvent to ''tert''-butyl alcohol. Production TAA is primarily made by the hydration of 2-methyl-2-butene in the presence of an acidic catalyst. Natural occurrence Fusel alcohols like TAA are grain fermentation byproducts and therefore trace amounts of TAA are present in many alcoholic beverages. Traces of TAA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |