|
1,1,2-Trifluoroethane
1,1,2-Trifluoroethane or R-143, is a hydrofluorocarbon with formula . It is a colourless gas at room temperature. It is an asymmetrical isomer of 1,1,1-trifluoroethane. 1,1,2-Trifluoroethane has a global warming potential of 397 for 100 years.G. Myhre, D. Shindell et al.: Climate Change 2013: The Physical Science Basis. Working Group I contribution to the IPCC Fifth Assessment Report. Hrsg.: Intergovernmental Panel on Climate Change. 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, 24–39; Table 8.SM.16 1,1,2-Trifluoroethane can be obtained by the hydrogenation of 1,2-dichlorodifluoroethylene or chlorotrifluoroethylene. See also * 1,2-Dichloro-1,1,2-trifluoroethane * 1,1,2-Trichloro-1,2,2-trifluoroethane * 1,1,2-Trichloroethane 1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula CHCl. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroethylene
Trifluoroethylene (abbreviated as TrFE) is an organofluoride compound with the chemical formula . It is a colourless gas.Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-500 TrFE can polymerise to form poly(trifluoroethylene) (PTrFE). It can also form copolymers with other monomers, such as vinylidene fluoride to form a co-polymer that is used to produce ferroelectric material Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the add ...s. References {{reflist Monomers Fluoroalkenes Hydrofluoroolefins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-trifluoroethane
1,1,1-Trifluoroethane, or R-143a or simply trifluoroethane, is a hydrofluorocarbon (HFC) compound that is a colorless gas. It should not be confused with the much more commonly used HFC gas R-134a, nor confused with the isomeric compound 1,1,2-trifluoroethane. 1,1,1-Trifluoroethane has a critical temperature of 73 °C. Applications Trifluoroethane is used as a refrigerant either by itself or more commonly as a component of blended mixtures. It is also used as a propellant in canned air products used to clean electronic equipment. Environmental effects Unlike CFCs used as refrigerants, trifluoroethane has no chlorine atoms and therefore is not ozone-depleting. Its high chemical stability and infra-red absorbency make it a potent greenhouse gas with a lifetime of about 50 years and a global warming potential of 4300, which are at the high end compared to many other commonly used HFC refrigerants. Its abundance in the atmosphere more than doubled from about 10 parts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,2-Trichloroethane
1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula CHCl. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane. It is used as a solvent and as an intermediate in the synthesis of 1,1-dichloroethane. 1,1,2-TCA is a central nervous system depressant and inhalation of vapors may cause dizziness, drowsiness, headache, nausea, shortness of breath, and unconsciousness. Toxicology Trichloroethane may be harmful by inhalation, ingestion, and skin contact. It is a respiratory and eye irritant. Although no definitive studies currently exist, trichloroethane should be treated as a potential carcinogen since laboratory evidence suggests that low molecular weight chlorinated hydrocarbons may be carcinogenic. The Occupational Safety and Health Administration and National Institute for Occupational Safety and Health The National Institute f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluorocarbon
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection. They do not harm the ozone layer as much as the compounds they replace, but they do contribute to global warming, with trifluoromethane having 11,700 times the warming po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Global Warming Potential
Global warming potential (GWP) is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat that would be absorbed by the same mass of carbon dioxide (). GWP is 1 for . For other gases it depends on the gas and the time frame. Carbon dioxide equivalent (e or eq or -e) is calculated from GWP. For any gas, it is the mass of that would warm the earth as much as the mass of that gas. Thus it provides a common scale for measuring the climate effects of different gases. It is calculated as GWP times mass of the other gas. Methane has GWP (over 100 years) of 27.9 meaning that, for example, a leak of a tonne of methane is equivalent to emitting 27.9 tonnes of carbon dioxide. Similarly a tonne of nitrous oxide, from manure for example, is equivalent to 273 tonnes of carbon dioxide. Values Carbon dioxide is the reference. It has a GWP of 1 regardless of the time period used. emissions cause increases in atmospheric concentrations of that will last thousands ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dichlorodifluoroethylene
A dichlorodifluoroethylene (systematically named dichlorodifluoroethene) is one of three compounds with the chemical formula . Dichlorodifluoroethylenes are colourless gases, and are some of the simplest chlorodifluoroalkenes. The structural isomers are used as intermediates or precursors in the production of other industrial chemicals. 1,1-Dichloro-2,2-difluoroethylene 1,1-Dichloro-2,2-difluoroethylene is a low-boiling liquid that is used a refrigerant. It may also be used as a solvent, but has practical limitations as such, because of its low boiling point (commercial listings, 19 °C; lit. 17 °C). It is regarded as a hazardous chemical for being toxic by inhalation (see MSDS A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely ...), and a low-boiling liquid, and it c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. The thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dichlorination of the latter gives hexafluorocyclobutene Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dichloro-1,1,2-trifluoroethane
1,2-Dichloro-1,1,2-trifluoroethane is a volatile liquid chlorofluoroalkane composed of carbon, hydrogen, chlorine and fluorine, and with structural formula CClF2CHClF. It is also known as a refrigerant with the designation R-123a. Formation 1,1,2-Trichloro-1,2,2-trifluoroethane can be biotransformed in sewage sludge to 1,2-dichloro-1,1,2-trifluoroethane. Properties The critical temperature Critical or Critically may refer to: *Critical, or critical but stable, medical states **Critical, or intensive care medicine *Critical juncture, a discontinuous change studied in the social sciences. *Critical Software, a company specializing in ... of R-123a is . The rotation of the molecule appears to be hindered by the present of chlorine on each carbon atom, but is eased at higher temperatures. Use Although not deliberately used, R-123a is a significant impurity in its isomer, the widely used 2,2-dichloro-1,1,1-trifluoroethane (R-123). References External links * {{DEFAULTSORT:Dic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
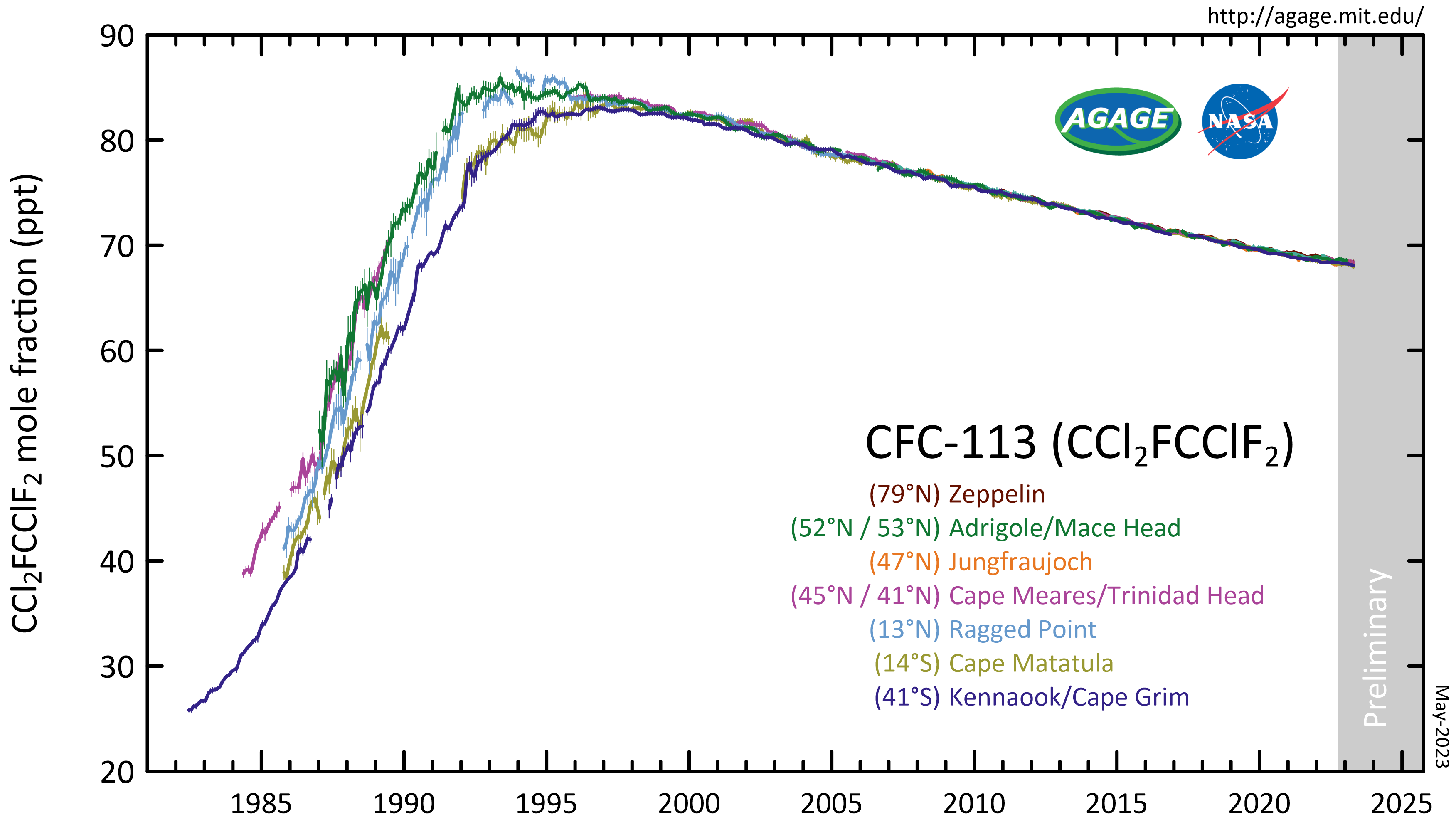
1,1,2-Trichloro-1,2,2-trifluoroethane
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It remains in the atmosphere about 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (where sunlight in the 190-225 nm (UV) range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• will destroy an average of 100,000 molecules during its atmospheric lifetime of 1–2 years. In some parts of the world, these reactions have significantly thinned the Earth's natural stratospheric ozone layer that shields the biosphere against solar UV radiation; increased UV ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluorocarbons
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection. They do not harm the ozone layer as much as the compounds they replace, but they do contribute to global warming, with trifluoromethane having 11,700 times the warming po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |