|
1,2-difluoroethane
1,2-Difluoroethane is a saturated hydrofluorocarbon containing an atom of fluorine attached to each of two carbons atoms. The formula can be written CH2FCH2F. It is an isomer of 1,1-difluoroethane. It has a HFC name of HFC-152 with no letter suffix. When cooled to cryogenic temperatures it can have different conformers, gauche and trans. In the liquid form these are about equally abundant and easily interconvert. As a gas it is mostly the gauche form. In the HFC-152 designation, 2 means two fluorine atoms, 5 means 5-1 or four hydrogen atoms, and 1 means 1+1 or two carbon atoms. Formation Ethylene reacts explosively with fluorine yielding a mixture of 1,2-difluoroethane and vinyl fluoride. With solid fluorine it will react when triggered by near-infrared radiation. Properties Critical temperature is 107.5 ô¯C. If a C-H bond is over excited with too much vibration, the intramolecular vibrational relaxation takes 490 picoseconds. The F-C-C-F dihedral angle is about 72ô ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluorocarbon
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection. They do not harm the ozone layer as much as the compounds they replace, but they do contribute to global warming, with trifluoromethane having 11,700 times the warming po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocitrate
Fluorocitric acid is a fluorinated carboxylic acid derived from citric acid by substitution of one hydrogen by a fluorine atom. The appropriate anion is called fluorocitrate. Fluorocitrate is formed in two steps from fluoroacetate. Fluoroacetate is first converted to fluoroacetyl-CoA by acetyl-CoA synthetase in the mitochondria. Then fluoroacetyl-CoA condenses with oxaloacetate to form fluorocitrate. This step is catalyzed by citrate synthase. Flurocitrate is a metabolite of fluoroacetic acid and is very toxic because it is not processable using aconitase in the citrate cycle (where fluorocitrate takes place of citrate as the substrate). The enzyme is inhibited and the cycle stops working. See also * Citric acid * Fluoroacetic acid * Citrate cycle The citric acid cycle (CAC)ãalso known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)ãis a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse gases in Atmosphere of Earth, Earth's atmosphere are water vapor (), carbon dioxide (), methane (), nitrous oxide (), and ozone (). Without greenhouse gases, the average temperature of Earth#Surface, Earth's surface would be about , rather than the present average of . The atmospheres of atmosphere of Venus, Venus, atmosphere of Mars, Mars and atmosphere of Titan, Titan also contain greenhouse gases. Human activities since the beginning of the Industrial Revolution (around 1750) have increased the Carbon dioxide in Earth's atmosphere, atmospheric concentration of carbon dioxide by over 50%, from 280 parts per million, ppm in 1750 to 421 ppm in 2022. The last time the atmospheric concentration of carbon dioxide was this high was over 3&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluoric Acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material polytetrafluoroethylene, PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to Etching (microfabrication), etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Polytetrafluoroethylene, Teflon, fluoropolymers, fluorocarbons, and refrigeration, refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formyl Fluoride
Formyl fluoride is the organic compound with the formula HC(O)F. Preparation HC(O)F was first reported in 1934. Among the many preparations, a typical one involves the reaction of sodium formate with benzoyl fluoride (generated in situ from KHF2 and benzoyl chloride): :HCOONa + C6H5C(O)F ã FC(O)H + C6H5COONa Structure The molecule is planar; C-O and C-F distances are 1.18 and 1.34 A, respectively. Reactions HC(O)F decomposes autocatalytically near room temperature to carbon monoxide and hydrogen fluoride: :HC(O)F ã HF + CO Because of the compound's sensitivity, reactions are conducted at low temperatures and samples are often stored over anhydrous alkali metal fluorides, e.g. potassium fluoride which absorbs HF. Benzene (and other arenes) react with formyl fluoride in the presence of boron trifluoride to give benzaldehyde. In a related reaction, formyl chloride is implicated in Gattermann-Koch formylation reaction. The reaction of formyl fluoride/BF3 with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxides
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide *Nitrogen dioxide (), nitrogen(IV) oxide *Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide *Dinitrogen dioxide (), nitrogen(II) oxide Dimer (chemistry), dimer *Dinitrogen trioxide (), nitrogen(II,IV) oxide *Dinitrogen tetroxide (), nitrogen(IV) oxide Dimer (chemistry), dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate *Nitrosyl azide (), nitrogen(ãI,0,I,II) oxide *Nitryl azide () *Oxatetrazole () *Trinitramide ( or ), nitrogen(0,IV) oxide Anions *Nitroxyl, Nitroxide () *Nitrite ( or ) *Nitrate () *Peroxynitrite ( or ) *Peroxynitrate ( or ) *Orthonitrate (, analogous to phosphate ) *Hyponitrite ( or ) *Trioxodinitrate or hyponitrate ( or ) *Nitroxylate ( or ) *Ammonium dinitramide, Dinitramide ( or ) Cations *Nitrosonium ( or ) *Nitronium ( or ) Atm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
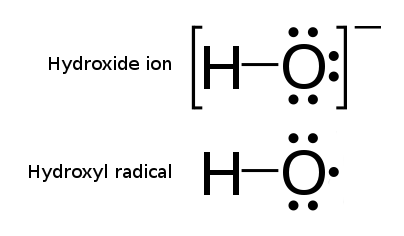
Hydroxyl Radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important in the field of radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of 1-hydroxy-2(1''H'')-pyridinethione. Notation The unpaired electron of the hydroxyl radical is officially represented by a middle dot, ãÂ, beside the O. Biology Hydroxyl radicals can occasionally be produced as a byproduct of immune action. Macrophages and microglia most frequently generate this compound when exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry's Law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century. An example where Henry's law is at play is in the depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that changes during decompression, leading to decompression sickness. An everyday example is given by one's experience with carbonated soft drinks, which contain dissolved carbon dioxide. Before opening, the gas above the drink in its container is almost pure carbon dioxide, at a pressure higher than atmospheric pressure. After the bottle is opened, this gas escapes, moving the partial pressure of carbon dioxide above the liquid to be much lower, resulting in degassing as the dissolved carbon dioxide comes out of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance (pharmacology), clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Ronald W. Estabrook, Estabrook, David Y. Cooper, Cooper, and Otto Rosenthal, Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of secondary metabolite, defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdom (biology), kingdoms of life: animals, plants, fungus, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-difluoroethane
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula CHF. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years). Production 1,1-Difluoroethane is a synthetic substance that is produced by the mercury-catalyzed addition of hydrogen fluoride to acetylene: :HCCH + 2 HF ã CHCHF The intermediate in this process is vinyl fluoride (C2H3F), the monomeric precursor to polyvinyl fluoride. Uses With a relatively low global warming potential (GWP) index of 124 and favorable thermophysical properties, 1,1-difluoroethane has been proposed as an environmentally friendly alternative to R134a. Despite its flammability, R152a also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroacetate
Fluoroacetate may refer to: * Fluoroacetic acid * Sodium fluoroacetate Sodium fluoroacetate is an organofluorine chemical compound with the formula FCH2CO2Na. This colourless salt has a taste similar to that of sodium chloride and is used as a rodenticide. History and production The effectiveness of sodium fluoroa ... {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_0468.jpg)