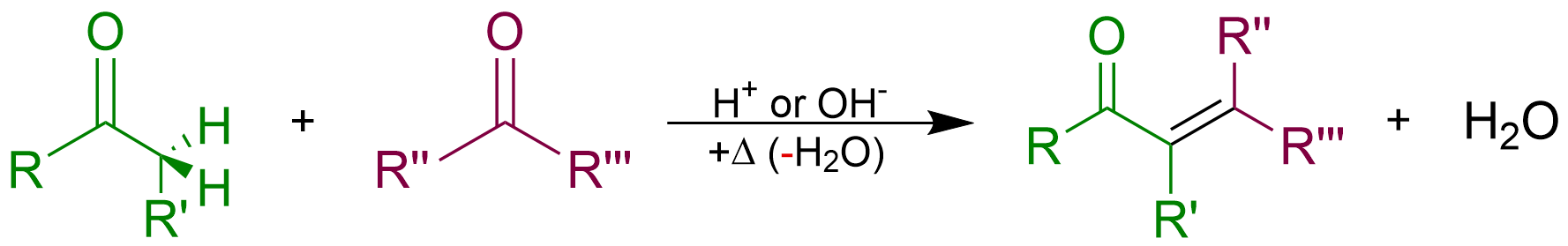|
α-Eucaine
α-Eucaine (''alpha''-eucaine) is a drug that was previously used as a local anesthetic. It was designed as an Functional analog (chemistry), analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic. Synthesis The Aldol condensation between two equivalents of acetone gives Mesityl oxide [141-79-7] (1) (isophorone is a side-product of this reaction). Ammonolysis of mesityl oxide formed diacetonamine [625-04-7] (2). The reaction of this product with acetone then gives 2,2,6,6-tetramethyl-4-piperidone [826-36-8] (3). ''N''-methylation of the secondary amine gives 1,2,2,6,6-pentamethylpiperidin-4-one [5554-54-1] (4). Cyanohydrin formation giveCID:434556(5). Esterification of the tertiary alcohol with benzoyl chloride gives (6). Pinner reaction of the nitrile with EtOH/H+ affords alpha-eucaine (7). See also * Eucaine, or β-eucaine, a related local anesthetic References Local anesthetics Piperidines Benzoate esters Methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eucaine (alpha) Synthesis
Eucaine, also known as β-eucaine or Betacaine, is a drug that was previously used as a local anesthetic. It was designed as an analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic. It is a white, crystalline solid. Prior to World War I, Britain imported eucaine from Germany. During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain. The brand name Betacaine can sometimes refer to a preparation containing lidocaine, not eucaine. Synthesis Condensation of diacetonamine 25-04-7(1) with acetaldehyde (paraldehyde) rather than acetone gives the piperidone containing one less methyl group, i.e. 2,2,6-trimethylpiperidin-4-one 311-23-7(2). Reduction of the ketone with sodium amalgam gives the alcohol as a mixture of isomers, 2,2,6-trimethylpiperidin-4-ol (3). Benzoylation then affords beta-eucaine (4). See also * α-Eucaine α-Eucaine (''alpha''-eucaine) is a dru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eucaine
Eucaine, also known as β-eucaine or Betacaine, is a drug that was previously used as a local anesthetic. It was designed as an Functional analog (chemistry), analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic. It is a white, crystalline solid. Prior to World War I, Britain imported eucaine from Germany. During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain. The brand name Betacaine can sometimes refer to a preparation containing lidocaine, not eucaine. Synthesis Condensation of diacetonamine [625-04-7] (1) with acetaldehyde (paraldehyde) rather than acetone gives the piperidone containing one less methyl group, i.e. 2,2,6-trimethylpiperidin-4-one [3311-23-7] (2). Reduction of the ketone with sodium amalgam gives the alcohol as a mixture of isomers, 2,2,6-trimethylpiperidin-4-ol (3). Benzoylation then affords beta-eucaine (4). See also * α-Eucaine, a related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways (local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical administration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Analog (chemistry)
In chemistry and pharmacology, functional analogs are chemical compounds that have similar physical, chemical, biochemical, or pharmacological properties. Functional analogs are not necessarily structural analogs with a similar chemical structure. An example of pharmacological functional analogs are morphine, heroin and fentanyl, which have the same mechanism of action, but fentanyl is structurally quite different from the other two with significant variance in dosage. File:Morphin - Morphine.svg, Morphine File:Heroin - Heroine.svg, Heroin File:Fentanyl2DCSD.svg, Fentanyl See also *Federal Analogue Act The Federal Analogue Act, , is a section of the United States Controlled Substances Act passed in 1986 which allows any chemical "substantially similar" to a controlled substance listed in Schedule I or II to be treated as if it were listed in ... References Chemical nomenclature Pharmacology {{pharma-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cocaine
Cocaine (from , from , ultimately from Quechuan languages, Quechua: ''kĂşka'') is a central nervous system (CNS) stimulant mainly recreational drug use, used recreationally for its euphoria, euphoric effects. It is primarily obtained from the leaves of two Coca species native to South America, ''Erythroxylum coca'' and ''Erythroxylum novogranatense''. After extraction from coca leaves and further processing into cocaine hydrochloride (powdered cocaine), the drug is often Insufflation (medicine), snorted, applied topical administration, topically to the mouth, or dissolved and injection (medicine), injected into a vein. It can also then be turned into free base form (crack cocaine), in which it can be heated until sublimated and then the vapours can be smoking, inhaled. Cocaine stimulates the mesolimbic pathway, reward pathway in the brain. Mental effects may include an euphoria, intense feeling of happiness, sexual arousal, psychosis, loss of contact with reality, or psychomo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesityl Oxide
Mesityl oxide is a Carbonyl#.CE.B1.2C.CE.B2-Unsaturated_carbonyl_compounds, α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor. Synthesis It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound. : Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition: : Phorone is formed by continued aldol condensation: : Uses Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation: : Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol). Dimedone is another established use of mesityl oxide. References {{reflist External linksIPCS INCHEM Description Enones Ketone solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone
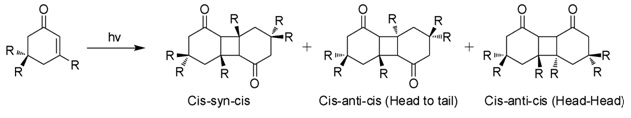
Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetics
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways (local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical administration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoate Esters
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |