|
(Trimethylsilyl)methyllithium
(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer iCH2tmssub>6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane i4(μ3-CH2tms)4(Et2O)2and Tetramethylethylenediamine_.html"_;"title="i2(μ-CH2tms)2(Tetramethylethylenediamine_">tmeda)2 _Preparation (Trimethylsilyl)methyllithium,_which_is_commercially_available_as_a_THF_solution,_is_usually_prepared_by_treatment_of_(Trimethylsilyl)methyl_chloride.html" ;"title="Tetramethylethylenediamine ">tmeda)2">Tetramethylethylenediamine_.html" ;"title="i2(μ-CH2tms)2(Tetramethylethylenediamine ">tmeda)2 Preparation (Trimethylsilyl)methyllithium, which is commercially available as a THF solution, is usually prepared by treatment of (Trimethylsilyl)methyl chloride">[(trimethylsilyl)methyl c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(Trimethylsilyl)methyl Chloride
(Trimethylsilyl)methyl chloride is the organosilicon compound with the formula (CH3)3SiCH2Cl. A colorless, volatile liquid, it is an alkylating agent that is employed in organic synthesis, especially as a precursor to (trimethylsilyl)methyllithium. In the presence of triphenylphosphine, it olefinates benzophenones: : See also * Trimethylsilyl chloride Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. I ..., a silyl chloride References {{DEFAULTSORT:Trimethylsilyl methyl chloride Organosilicon compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilylmethyl Chloride
(Trimethylsilyl)methyl chloride is the organosilicon compound with the formula (CH3)3SiCH2Cl. A colorless, volatile liquid, it is an alkylating agent that is employed in organic synthesis, especially as a precursor to (trimethylsilyl)methyllithium. In the presence of triphenylphosphine, it olefinates benzophenones: : See also * Trimethylsilyl chloride Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. I ..., a silyl chloride References {{DEFAULTSORT:Trimethylsilyl methyl chloride Organosilicon compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neopentyl
Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane. In older literature, the common non-systematic name amyl was often used for the pentyl group. Conversely, the name pentyl was used for several five-carbon branched alkyl groups, distinguished by various prefixes. The nomenclature has now reversed, with "amyl" being more often used to refer to the terminally branched group also called isopentyl, as in amobarbital. A cyclopentyl group is a ring with the formula -C5H9. The name is also used for the pentyl radical, a pentyl group as an isolated molecule. This free radical is only observed in extreme conditions. Its formula is often written "•" or "• " to indicate that it has one unsatisfied valence bond. Older "pentyl" groups The following names are still used sometimes: Pentyl radical The free radical pentyl was studied by J. Pacansky and A. Gutierrez in 1983. The radical was obtained by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-hydride Elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site ''cis'' to the alkyl group for this reaction to occur. Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0 metals alkyls are generally more stable to β-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available. The β-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process relies on β-hydride elimination to produce α-olefins which are used to produce detergents. Illustrative of a sometimes undesirable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Chloride
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive. Bonding Halides are X-type ligands in coordination chemistry. They are both σ- and π-donors. Chloride is commonly found as both a terminal ligand and a bridging ligand. The halide ligands are weak field ligands. Due to a smaller crystal field splitting energy, the homoleptic halide complexes of the first transition series are all high spin. Only rCl6sup>3− is exchange inert. Homoleptic metal halide complexes are known with several stoichiometries, but the main ones are the hexahalometallates and the tetrahalometallates. The hexahalides adopt octahedral coordination geometry, whereas the tetrahalides are usually tetrahedral. Square planar tetrahalides are known for Pd(II), Pt(II), and Au(III). Examples with 2- and 3-coordination are common for Au(I), Cu(I), and Ag(I). Due to the presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt Metathesis
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme: :AB + CD -> AD + CB The bond between the reacting species can be either ionic or covalent. Classically, these reactions result in the precipitation of one product. In older literature, the term double decomposition is frequently encountered. The term double decomposition is more specifically used when at least one of the substances does not dissolve in the solvent, as the ligand or ion exchange takes place in the solid state of the reactant. For example: :AX(aq) + BY(s) → AY(aq) + BX(s). Types of reactions Counterion exchange Salt metathesis is a common technique for exchanging counterions. The choice of reactants is guided by a solubility chart or lattice energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
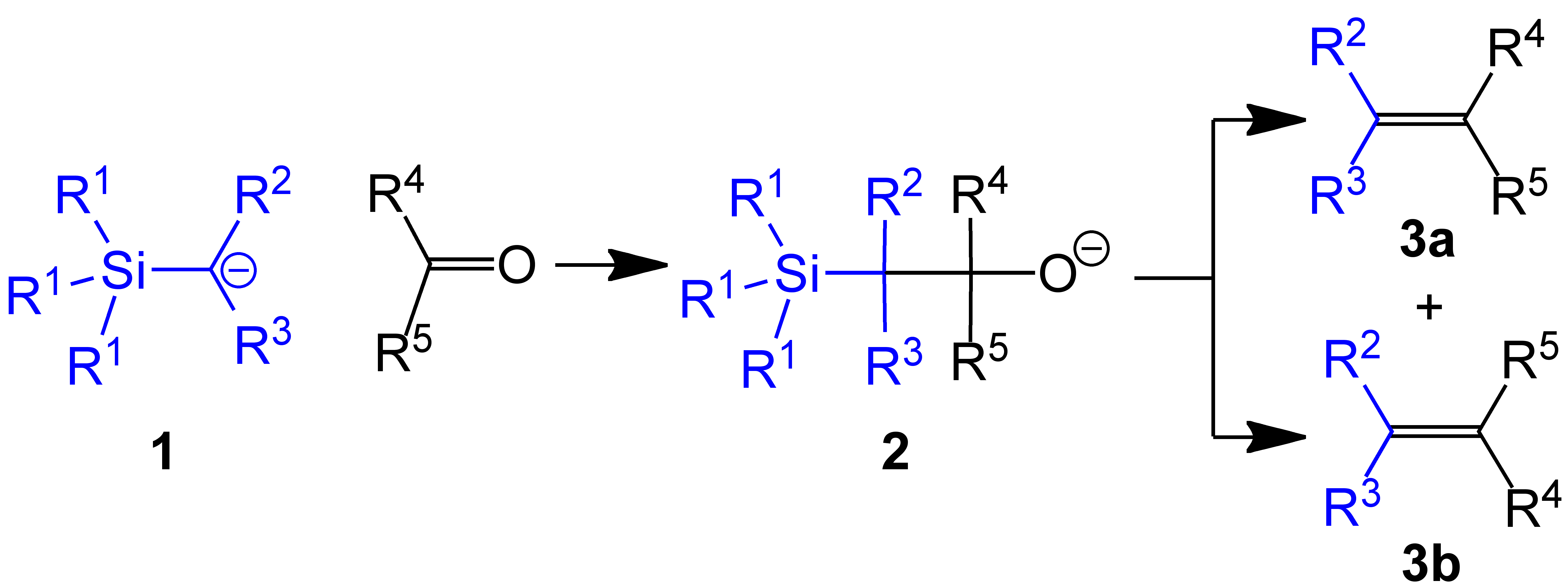
Petersen Olefination Scheme V
Petersen is a common Danish patronymic surname, meaning ''"son of Peter"''. There are other spellings. Petersen may refer to: People In arts and entertainment * Adolf Dahm-Petersen, Norwegian voice specialist * Anja Petersen, German operatic soprano and university lecturer * Anker Eli Petersen, Faroese writer and artist * Ann Petersen, Belgian actress * Chris Petersen (born 1963), American child actor * Devon Petersen (born 1986), South African darts player * Elmer Petersen, American artist * Gustaf Munch-Petersen, Danish writer and painter * Joel Petersen, bass guitarist * John Hahn-Petersen, Danish actor * Josef Petersen, Danish novelist * Patrick Petersen, American actor * Paul Petersen, American movie actor, singer, novelist, and activist * Robert E. Petersen, publisher, auto museum founder * Robert Storm Petersen, Danish cartoonist, writer, animator, illustrator, painter and humorist * Sandy Petersen, American game designer * Uwe Fahrenkrog-Petersen, German musicia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |