Wave–particle Duality on:
[Wikipedia]
[Google]
[Amazon]
Wave–particle duality is the concept in
 Electrons from the source hit a wall with two thin slits. A mask behind the slits can expose either one or open to expose both slits. The results for high electron intensity are shown on the right, first for each slit individually, then with both slits open. With either slit open there is a smooth intensity variation due to diffraction. When both slits are open the intensity oscillates, characteristic of wave interference.
Having observed wave behavior, now change the experiment, lowering the intensity of the electron source until only one or two are detected per second, appearing as individual particles, dots in the video. As shown in the movie clip below, the dots on the detector seem at first to be random. After some time a pattern emerges, eventually forming an alternating sequence of light and dark bands.
The experiment shows wave interference revealed a single particle at a time—quantum mechanical electrons display both wave and particle behavior. Similar results have been shown for atoms and even large molecules.
Electrons from the source hit a wall with two thin slits. A mask behind the slits can expose either one or open to expose both slits. The results for high electron intensity are shown on the right, first for each slit individually, then with both slits open. With either slit open there is a smooth intensity variation due to diffraction. When both slits are open the intensity oscillates, characteristic of wave interference.
Having observed wave behavior, now change the experiment, lowering the intensity of the electron source until only one or two are detected per second, appearing as individual particles, dots in the video. As shown in the movie clip below, the dots on the detector seem at first to be random. After some time a pattern emerges, eventually forming an alternating sequence of light and dark bands.
The experiment shows wave interference revealed a single particle at a time—quantum mechanical electrons display both wave and particle behavior. Similar results have been shown for atoms and even large molecules.
 While electrons were thought to be particles until their wave properties were discovered, for photons it was the opposite. In 1887,
While electrons were thought to be particles until their wave properties were discovered, for photons it was the opposite. In 1887,  where ''h'' is the
where ''h'' is the
 A laser beam along the input port splits at a half-silvered mirror. Part of the beam continues straight, passes though a glass
A laser beam along the input port splits at a half-silvered mirror. Part of the beam continues straight, passes though a glass
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
that fundamental entities of the universe, like photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s and electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, exhibit particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
or wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
properties according to the experimental circumstances. It expresses the inability of the classical concepts such as particle or wave to fully describe the behavior of quantum objects. During the 19th and early 20th centuries, light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
was found to behave as a wave then later was discovered to have a particle-like behavior, whereas electrons behaved like particles in early experiments then were later discovered to have wave-like behavior. The concept of duality arose to name these seeming contradictions.
History
Wave-particle duality of light
In the late 17th century, SirIsaac Newton
Sir Isaac Newton () was an English polymath active as a mathematician, physicist, astronomer, alchemist, theologian, and author. Newton was a key figure in the Scientific Revolution and the Age of Enlightenment, Enlightenment that followed ...
had advocated that light was corpuscular (particulate), but Christiaan Huygens
Christiaan Huygens, Halen, Lord of Zeelhem, ( , ; ; also spelled Huyghens; ; 14 April 1629 – 8 July 1695) was a Dutch mathematician, physicist, engineer, astronomer, and inventor who is regarded as a key figure in the Scientific Revolution ...
took an opposing wave description. While Newton had favored a particle approach, he was the first to attempt to reconcile both wave and particle theories of light, and the only one in his time to consider both, thereby anticipating modern wave-particle duality. Thomas Young's interference experiments in 1801, and François Arago
Dominique François Jean Arago (), known simply as François Arago (; Catalan: , ; 26 February 17862 October 1853), was a French mathematician, physicist, astronomer, freemason, supporter of the Carbonari revolutionaries and politician.
Early l ...
's detection of the Poisson spot in 1819, validated Huygens' wave models. However, the wave model was challenged in 1901 by Planck's law
In physics, Planck's law (also Planck radiation law) describes the spectral density of electromagnetic radiation emitted by a black body in thermal equilibrium at a given temperature , when there is no net flow of matter or energy between the ...
for black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
. Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
heuristically derived a formula for the observed spectrum by assuming that a hypothetical electrically charged oscillator
Oscillation is the repetitive or periodic variation, typically in time, of some measure about a central value (often a point of equilibrium) or between two or more different states. Familiar examples of oscillation include a swinging pendulum ...
in a cavity that contained black-body radiation could only change its energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
in a minimal increment, ''E'', that was proportional to the frequency of its associated electromagnetic wave
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength, ...
. In 1905 Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
interpreted the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
also with discrete energies for photons. These both indicate particle behavior. Despite confirmation by various experimental observations, the photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
theory (as it came to be called) remained controversial until Arthur Compton
Arthur Holly Compton (September 10, 1892 – March 15, 1962) was an American particle physicist who won the 1927 Nobel Prize in Physics for his discovery of the Compton effect, which demonstrated the particle nature of electromagnetic radiati ...
performed a series of experiments from 1922 to 1924 demonstrating the momentum of light. The experimental evidence of particle-like momentum and energy seemingly contradicted the earlier work demonstrating wave-like interference of light.
Wave-particle duality of matter
The contradictory evidence from electrons arrived in the opposite order. Many experiments byJ. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was an English physicist who received the Nobel Prize in Physics in 1906 "in recognition of the great merits of his theoretical and experimental investigations on the conduction of ...
, Robert Millikan
Robert Andrews Millikan ( ; March 22, 1868 – December 19, 1953) was an American physicist who received the Nobel Prize in Physics in 1923 "for his work on the elementary charge of electricity and on the photoelectric effect".
Millikan gradua ...
, and Charles Wilson among others had shown that free electrons had particle properties, for instance, the measurement of their mass by Thomson in 1897. In 1924, Louis de Broglie
Louis Victor Pierre Raymond, 7th Duc de Broglie (15 August 1892 – 19 March 1987) was a French theoretical physicist and aristocrat known for his contributions to quantum theory. In his 1924 PhD thesis, he postulated the wave nature of elec ...
introduced his theory of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
waves in his PhD thesis ''Recherches sur la théorie des quanta''. He suggested that an electron around a nucleus could be thought of as being a standing wave
In physics, a standing wave, also known as a stationary wave, is a wave that oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with respect t ...
and that electrons and all matter could be considered as waves. He merged the idea of thinking about them as particles, and of thinking of them as waves. He proposed that particles are bundles of waves (wave packet
In physics, a wave packet (also known as a wave train or wave group) is a short burst of localized wave action that travels as a unit, outlined by an Envelope (waves), envelope. A wave packet can be analyzed into, or can be synthesized from, a ...
s) that move with a group velocity
The group velocity of a wave is the velocity with which the overall envelope shape of the wave's amplitudes—known as the ''modulation'' or ''envelope (waves), envelope'' of the wave—propagates through space.
For example, if a stone is thro ...
and have an effective mass. Both of these depend upon the energy, which in turn connects to the wavevector
In physics, a wave vector (or wavevector) is a vector used in describing a wave, with a typical unit being cycle per metre. It has a magnitude and direction. Its magnitude is the wavenumber of the wave (inversely proportional to the wavelength), ...
and the relativistic formulation of Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
a few years before.
Following de Broglie's proposal of wave–particle duality of electrons, in 1925 to 1926, Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
developed the wave equation of motion for electrons. This rapidly became part of what was called by Schrödinger ''undulatory mechanics'', now called the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
and also "wave mechanics".
In 1926, Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
gave a talk in an Oxford meeting about using the electron diffraction experiments to confirm the wave–particle duality of electrons. In his talk, Born cited experimental data from Clinton Davisson
Clinton Joseph Davisson (October 22, 1881 – February 1, 1958) was an American physicist who shared the 1937 Nobel Prize in Physics with George Paget Thomson "for their experimental discovery of the diffraction of electrons by crystals".
Earl ...
in 1923. It happened that Davisson also attended that talk. Davisson returned to his lab in the US to switch his experimental focus to test the wave property of electrons.
In 1927, the wave nature of electrons was empirically confirmed by two experiments. The Davisson–Germer experiment
The Davisson–Germer experiment was a 1923–1927 experiment by Clinton Davisson and Lester Germer at Bell Labs, Western Electric (later Bell Labs), in which electrons, scattered by the surface of a crystal of nickel metal, displayed a diffrac ...
at Bell Labs measured electrons scattered from Ni metal surfaces. George Paget Thomson
Sir George Paget Thomson (; 3 May 1892 – 10 September 1975) was an English physicist who shared the 1937 Nobel Prize in Physics with Clinton Davisson “for their experimental discovery of the diffraction of electrons by crystals”.
Educa ...
and Alexander Reid at Cambridge University scattered electrons through thin nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
films and observed concentric diffraction rings. Alexander Reid, who was Thomson's graduate student, performed the first experiments, but he died soon after in a motorcycle accident and is rarely mentioned. These experiments were rapidly followed by the first non-relativistic diffraction model for electrons by Hans Bethe
Hans Albrecht Eduard Bethe (; ; July 2, 1906 – March 6, 2005) was a German-American physicist who made major contributions to nuclear physics, astrophysics, quantum electrodynamics and solid-state physics, and received the Nobel Prize in Physi ...
based upon the Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
, which is very close to how electron diffraction is now described. Significantly, Davisson and Germer noticed that their results could not be interpreted using a Bragg's law
In many areas of science, Bragg's law — also known as Wulff–Bragg's condition or Laue–Bragg interference — is a special case of Laue diffraction that gives the angles for coherent scattering of waves from a large crystal lattice. It descr ...
approach as the positions were systematically different; the approach of Bethe, which includes the refraction due to the average potential, yielded more accurate results. Davisson and Thomson were awarded the Nobel Prize in 1937 for experimental verification of wave property of electrons by diffraction experiments. Similar crystal diffraction experiments were carried out by Otto Stern
:''Otto Stern was also the pen name of German women's rights activist Louise Otto-Peters (1819–1895)''.
Otto Stern (; 17 February 1888 – 17 August 1969) was a German-American physicist. He is the second most nominated person for a Nobel Pri ...
in the 1930s using beams of helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
atoms and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
molecules. These experiments further verified that wave behavior is not limited to electrons and is a general property of matter on a microscopic scale.
Classical waves and particles
Before proceeding further, it is critical to introduce some definitions of waves and particles both in a classical sense and in quantum mechanics. Waves and particles are two very different models for physical systems, each with an exceptionally large range of application. Classical waves obey thewave equation
The wave equation is a second-order linear partial differential equation for the description of waves or standing wave fields such as mechanical waves (e.g. water waves, sound waves and seismic waves) or electromagnetic waves (including light ...
; they have continuous values at many points in space that vary with time; their spatial extent can vary with time due to diffraction
Diffraction is the deviation of waves from straight-line propagation without any change in their energy due to an obstacle or through an aperture. The diffracting object or aperture effectively becomes a secondary source of the Wave propagation ...
, and they display wave interference
In physics, interference is a phenomenon in which two coherent waves are combined by adding their intensities or displacements with due consideration for their phase difference. The resultant wave may have greater amplitude (constructive in ...
. Physical systems exhibiting wave behavior and described by the mathematics of wave equations include water waves
In fluid dynamics, a wind wave, or wind-generated water wave, is a surface wave that occurs on the free surface of bodies of water as a result of the wind blowing over the water's surface. The contact distance in the direction of the wind is k ...
, seismic waves
A seismic wave is a mechanical wave of acoustic wave, acoustic energy that travels through the Earth or another planetary body. It can result from an earthquake (or generally, a quake (natural phenomenon), quake), types of volcanic eruptions ...
, sound waves
In physics, sound is a vibration that propagates as an acoustic wave through a transmission medium such as a gas, liquid or solid.
In human physiology and psychology, sound is the ''reception'' of such waves and their ''perception'' by the br ...
, radio waves
Radio waves (formerly called Hertzian waves) are a type of electromagnetic radiation with the lowest frequencies and the longest wavelengths in the electromagnetic spectrum, typically with frequencies below 300 gigahertz (GHz) and wavelengths ...
, and more.
Classical particles obey classical mechanics
Classical mechanics is a Theoretical physics, physical theory describing the motion of objects such as projectiles, parts of Machine (mechanical), machinery, spacecraft, planets, stars, and galaxies. The development of classical mechanics inv ...
; they have some center of mass
In physics, the center of mass of a distribution of mass in space (sometimes referred to as the barycenter or balance point) is the unique point at any given time where the weight function, weighted relative position (vector), position of the d ...
and extent; they follow trajectories
A trajectory or flight path is the path that an object with mass in motion follows through space as a function of time. In classical mechanics, a trajectory is defined by Hamiltonian mechanics via canonical coordinates; hence, a complete traje ...
characterized by positions and velocities that vary over time; in the absence of forces
In physics, a force is an influence that can cause an object to change its velocity unless counterbalanced by other forces. In mechanics, force makes ideas like 'pushing' or 'pulling' mathematically precise. Because the magnitude and directi ...
their trajectories are straight lines. Stars
A star is a luminous spheroid of plasma held together by self-gravity. The nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night; their immense distances from Earth make them appear as fixed points of ...
, planets
A planet is a large, rounded astronomical body that is generally required to be in orbit around a star, stellar remnant, or brown dwarf, and is not one itself. The Solar System has eight planets by the most restrictive definition of the te ...
, spacecraft
A spacecraft is a vehicle that is designed spaceflight, to fly and operate in outer space. Spacecraft are used for a variety of purposes, including Telecommunications, communications, Earth observation satellite, Earth observation, Weather s ...
, tennis balls
A tennis ball is a small, hollow ball used in games of tennis and real tennis. Tennis balls are fluorescent yellow in professional competitions, but in recreational play other colors are also used. Tennis balls are covered in a fibrous felt, w ...
, bullets
A bullet is a Kinetic energy weapon, kinetic projectile, a component of firearm ammunition that is Shooting, shot from a gun barrel. They are made of a variety of materials, such as copper, lead, steel, polymer, rubber and even wax; and are made ...
, sand grain
Sand is a granular material composed of finely divided mineral particles. Sand has various compositions but is usually defined by its grain size. Sand grains are smaller than gravel and coarser than silt. Sand can also refer to a textural cl ...
s: particle models work across a huge scale. Unlike waves, particles do not exhibit interference.
Some experiments on quantum systems show wave-like interference and diffraction; some experiments show particle-like collisions.
Quantum systems obey wave equations that predict particle probability distributions. These particles are associated with discrete values called quanta for properties such as spin
Spin or spinning most often refers to:
* Spin (physics) or particle spin, a fundamental property of elementary particles
* Spin quantum number, a number which defines the value of a particle's spin
* Spinning (textiles), the creation of yarn or thr ...
, electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
and magnetic moment
In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude ...
. These particles arrive one at time, randomly, but build up a pattern. The probability that experiments will measure particles at a point in space is the square of a complex-number valued wave. Experiments can be designed to exhibit diffraction and interference of the probability amplitude
In quantum mechanics, a probability amplitude is a complex number used for describing the behaviour of systems. The square of the modulus of this quantity at a point in space represents a probability density at that point.
Probability amplitu ...
. Thus statistically large numbers of the random particle appearances can display wave-like properties. Similar equations govern collective excitations called quasiparticle
In condensed matter physics, a quasiparticle is a concept used to describe a collective behavior of a group of particles that can be treated as if they were a single particle. Formally, quasiparticles and collective excitations are closely relate ...
s.
Electrons behaving as waves and particles
The electron double slit experiment is a textbook demonstration of wave-particle duality. A modern version of the experiment is shown schematically in the figure below. Electrons from the source hit a wall with two thin slits. A mask behind the slits can expose either one or open to expose both slits. The results for high electron intensity are shown on the right, first for each slit individually, then with both slits open. With either slit open there is a smooth intensity variation due to diffraction. When both slits are open the intensity oscillates, characteristic of wave interference.
Having observed wave behavior, now change the experiment, lowering the intensity of the electron source until only one or two are detected per second, appearing as individual particles, dots in the video. As shown in the movie clip below, the dots on the detector seem at first to be random. After some time a pattern emerges, eventually forming an alternating sequence of light and dark bands.
The experiment shows wave interference revealed a single particle at a time—quantum mechanical electrons display both wave and particle behavior. Similar results have been shown for atoms and even large molecules.
Electrons from the source hit a wall with two thin slits. A mask behind the slits can expose either one or open to expose both slits. The results for high electron intensity are shown on the right, first for each slit individually, then with both slits open. With either slit open there is a smooth intensity variation due to diffraction. When both slits are open the intensity oscillates, characteristic of wave interference.
Having observed wave behavior, now change the experiment, lowering the intensity of the electron source until only one or two are detected per second, appearing as individual particles, dots in the video. As shown in the movie clip below, the dots on the detector seem at first to be random. After some time a pattern emerges, eventually forming an alternating sequence of light and dark bands.
The experiment shows wave interference revealed a single particle at a time—quantum mechanical electrons display both wave and particle behavior. Similar results have been shown for atoms and even large molecules.
Observing photons as particles
Heinrich Hertz
Heinrich Rudolf Hertz (; ; 22 February 1857 – 1 January 1894) was a German physicist who first conclusively proved the existence of the electromagnetic waves predicted by James Clerk Maxwell's equations of electromagnetism.
Biography
Heinri ...
observed that when light with sufficient frequency hits a metallic surface, the surface emits cathode rays
Cathode rays are streams of electrons observed in vacuum tube, discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitte ...
, what are now called electrons. In 1902, Philipp Lenard
Philipp Eduard Anton von Lenard (; ; 7 June 1862 – 20 May 1947) was a Hungarian-German physicist who received the Nobel Prize in Physics in 1905 "for his work on cathode rays" and the discovery of many of their properties. One of his most im ...
discovered that the maximum possible energy of an ejected electron is unrelated to its intensity
Intensity may refer to:
In colloquial use
* Strength (disambiguation)
*Amplitude
* Level (disambiguation)
* Magnitude (disambiguation)
In physical sciences
Physics
*Intensity (physics), power per unit area (W/m2)
*Field strength of electric, m ...
. This observation is at odds with classical electromagnetism, which predicts that the electron's energy should be proportional to the intensity of the incident radiation. Alt URL. In 1905, Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
suggested that the energy of the light must occur a finite number of energy quanta. translated into English as The term "photon" was introduced in 1926. He postulated that electrons can receive energy from an electromagnetic field only in discrete units (quanta or photons): an amount of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
''E'' that was related to the frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
''f'' of the light by
:
Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
(6.626×10−34 J⋅s). Only photons of a high enough frequency (above a certain ''threshold'' value which, when multiplied by the Planck constant, is the work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
) could knock an electron free. For example, photons of blue light had sufficient energy to free an electron from the metal he used, but photons of red light did not. One photon of light above the threshold frequency could release only one electron; the higher the frequency of a photon, the higher the kinetic energy of the emitted electron, but no amount of light below the threshold frequency could release an electron. Despite confirmation by various experimental observations, the photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
theory (as it came to be called later) remained controversial until Arthur Compton
Arthur Holly Compton (September 10, 1892 – March 15, 1962) was an American particle physicist who won the 1927 Nobel Prize in Physics for his discovery of the Compton effect, which demonstrated the particle nature of electromagnetic radiati ...
performed a series of experiments from 1922 to 1924 demonstrating the momentum of light.
Both discrete (quantized) energies and also momentum are, classically, particle attributes. There are many other examples where photons display particle-type properties, for instance in solar sail
Solar sails (also known as lightsails, light sails, and photon sails) are a method of spacecraft propulsion using radiation pressure exerted by sunlight on large surfaces. A number of spaceflight missions to test solar propulsion and navigati ...
s, where sunlight could propel a space vehicle and laser cooling
Laser cooling includes several techniques where atoms, molecules, and small mechanical systems are cooled with laser light. The directed energy of lasers is often associated with heating materials, e.g. laser cutting, so it can be counterintuit ...
where the momentum is used to slow down (cool) atoms. These are a different aspect of wave-particle duality.
Which slit experiments
In a "which way" experiment, particle detectors are placed at the slits to determine which slit the electron traveled through. When these detectors are inserted, quantum mechanics predicts that the interference pattern disappears because the detected part of the electron wave has changed (loss ofcoherence
Coherence is, in general, a state or situation in which all the parts or ideas fit together well so that they form a united whole.
More specifically, coherence, coherency, or coherent may refer to the following:
Physics
* Coherence (physics ...
). Many similar proposals
Proposal(s) or The Proposal may refer to:
* Proposal (business)
* Research proposal
* Marriage proposal
* Proposition, a proposal in logic and philosophy
Arts, entertainment, and media
* ''The Proposal'' (album), an album by Ransom & Statik Se ...
have been made and many have been converted into experiments and tried out. Every single one shows the same result: as soon as electron trajectories are detected, interference disappears.
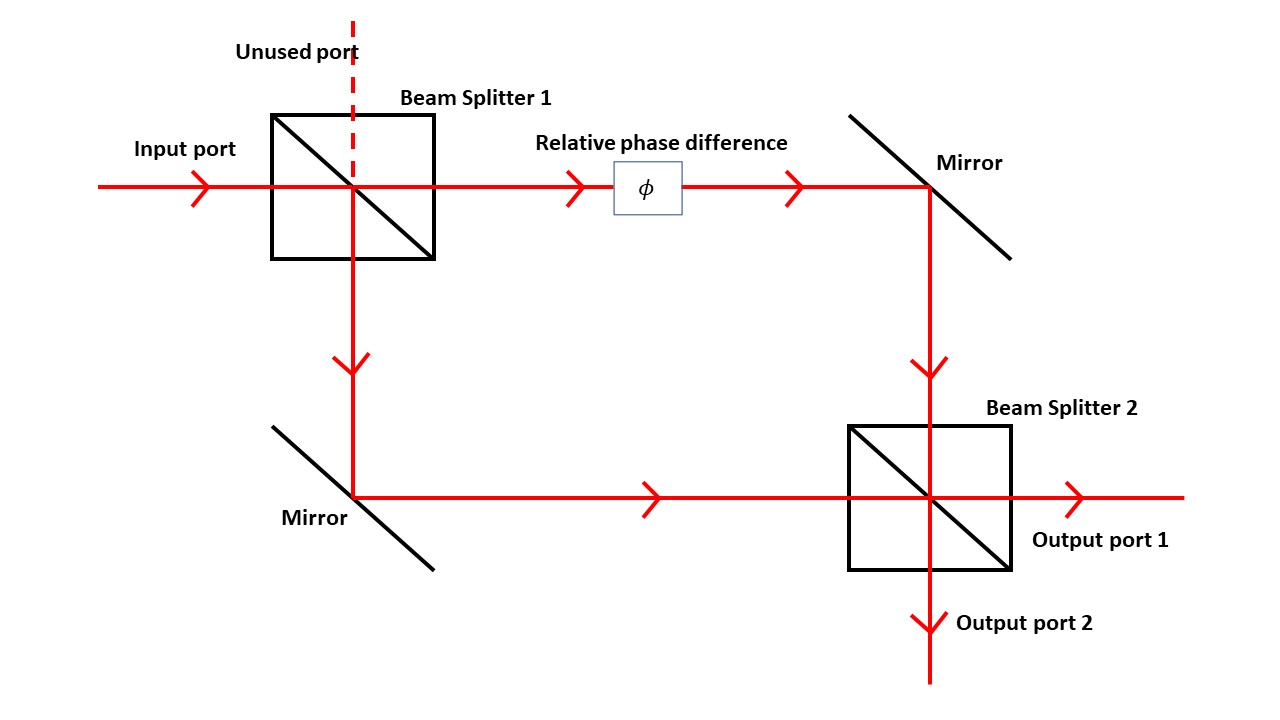
A simple example of these "which way" experiments uses a Mach–Zehnder interferometer
The Mach–Zehnder interferometer is a device used to determine the relative phase shift variations between two collimated beams derived by splitting light from a single source. The interferometer has been used, among other things, to measure p ...
, a device based on lasers and mirrors sketched below.
 A laser beam along the input port splits at a half-silvered mirror. Part of the beam continues straight, passes though a glass
A laser beam along the input port splits at a half-silvered mirror. Part of the beam continues straight, passes though a glass phase shifter
A phase shift module is a microwave network module which provides a controllable phase shift of the RF signal. Phase shifters are used in phased arrays.
Classification
Active versus passive
Active phase shifters provide gain, while passive ...
, then reflects downward. The other part of the beam reflects from the first mirror then turns at another mirror. The two beams meet at a second half-silvered beam splitter.
Each output port has a camera to record the results. The two beams show interference characteristic of wave propagation. If the laser intensity is turned sufficiently low, individual dots appear on the cameras, building up the pattern as in the electron example.
The first beam-splitter mirror acts like double slits, but in the interferometer case we can remove the second beam splitter. Then the beam heading down ends up in output port 1: any photon particles on this path gets counted in that port. The beam going across the top ends up on output port 2. In either case the counts will track the photon trajectories. However, as soon as the second beam splitter is removed the interference pattern disappears.
See also
* * * Einstein's thought experiments *Interpretations of quantum mechanics
An interpretation of quantum mechanics is an attempt to explain how the mathematical theory of quantum mechanics might correspond to experienced reality. Quantum mechanics has held up to rigorous and extremely precise tests in an extraordinarily b ...
*
* Uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
* Matter wave
Matter waves are a central part of the theory of quantum mechanics, being half of wave–particle duality. At all scales where measurements have been practical, matter exhibits wave-like behavior. For example, a beam of electrons can be diffract ...
* Corpuscular theory of light
In optics, the corpuscular theory of light states that light is made up of small discrete particles called " corpuscles" (little particles) which travel in a straight line with a finite velocity and possess impetus. This notion was based on an al ...
References
External links
* * * {{DEFAULTSORT:Wave Particle Duality Articles containing video clips Dichotomies Foundational quantum physics Waves Particles