Tafel Equation on:
[Wikipedia]
[Google]
[Amazon]
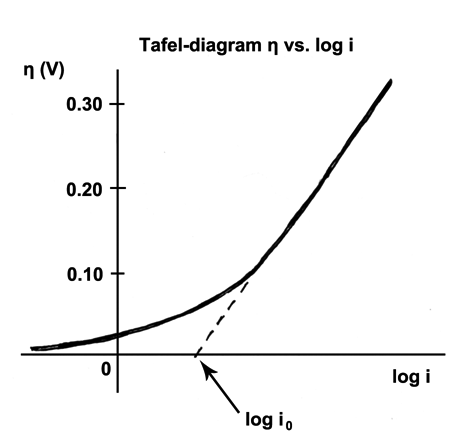 The Tafel equation is an equation in electrochemical kinetics relating the rate of an
The Tafel equation is an equation in electrochemical kinetics relating the rate of an
 The Tafel equation is an equation in electrochemical kinetics relating the rate of an
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase (typi ...
reaction to the overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly r ...
. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification. The equation is named after Swiss chemist Julius Tafel
Julius Tafel (2 June 1862 – 2 September 1918) was a Swiss chemist and electrochemist.
Early life and education
Julius Tafel was born in the village of Choindez in Courrendlin, Switzerland on 2 June 1862. Tafel's father, Julius Tafel Sr. ...
.It describes how the electrical current through an electrode depends on the voltage difference between the electrode and the bulk electrolyte for a simple, unimolecular redox reaction. :Where an electrochemical reaction occurs in two
half reaction
In chemistry, a half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the r ...
s on separate electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s, the Tafel equation is applied to each electrode separately. On a single electrode the Tafel equation can be stated as:
where
* the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction,
* : overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly r ...
, * : " Tafel slope", * : current density
In electromagnetism, current density is the amount of charge per unit time that flows through a unit area of a chosen cross section. The current density vector is defined as a vector whose magnitude is the electric current per cross-sectional ...
, /m2* : " exchange current density", /m2
A verification plus further explanation for this equation can be found here. The Tafel equation is an approximation of the Butler–Volmer equation in the case of . "Also, at a given electrode the Tafel equation assumes that the reverse half reaction rate is negligible compared to the forward reaction rate.The Tafel equation ''The'' is a grammatical article in English, denoting nouns that are already or about to be mentioned, under discussion, implied or otherwise presumed familiar to listeners, readers, or speakers. It is the definite article in English. ''The ...assumes that the concentrations at the electrode are practically equal to the concentrations in the bulk electrolyte, allowing the current to be expressed as a function of potential only. In other words, it assumes that the electrode mass transfer rate is much greater than the reaction rate, and that the reaction is dominated by the slower chemical reaction rate ".
Overview of the terms
The exchange current is the current at equilibrium, i.e. the rate at which oxidized and reduced species transfer electrons with the electrode. In other words, the exchange current density is the rate of reaction at the reversible potential (when the overpotential is zero by definition). At the reversible potential, the reaction is in equilibrium meaning that the forward and reverse reactions progress at the same rates. This rate is the exchange current density. The Tafel slope is measured experimentally. It can, however, be shown theoretically that when the dominant reaction mechanism involves the transfer of a single electron that where A is defined as where * * is theBoltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
,
* is the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
,
* is the electric elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
of an electron,
* is the thermal voltage
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the molar gas ...
, and
* is the charge transfer coefficient, the value of which must be between 0 and 1.
Equation in case of non-negligible electrode mass transfer
In a more general case,The following derivation of the extended Butler–Volmer equation is adapted from that of Bard and Faulkner and Newman and Thomas-Alyea. ... the current is expressed as a function not only of potential (as in the simple version), but of the given concentrations as well. The mass-transfer rate may be relatively small, but its only effect on the chemical reaction is through the altered (given) concentrations. In effect, the concentrations are a function of the potential as well.The Tafel equation can be also written as: where * ''n'' is the number of electrons exchanged, like in the
Nernst equation
In electrochemistry, the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction ( half-cell or full cell reaction) from the standard electrode potential, absolute tempera ...
,
* ''k'' is the rate constant
In chemical kinetics, a reaction rate constant or reaction rate coefficient () is a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants.
For a reaction between ...
for the electrode reaction in s−1,
* ''F'' is the Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
,
* ''C'' is the reactive species concentration at the electrode surface in mol/m2,
* the plus sign under the exponent refers to an anodic reaction, and a minus sign to a cathodic reaction,
* ''R'' is the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature, temperature ...
.
* is the charge transfer coefficient, the value of which must be between 0 and 1.
Demonstration
As seen in equation (), so: as seen in equation () and because . because due to the electrode mass transfer , which finally yields equation ().Equation in case of low values of polarization
An other equation is applicable at low values of polarization . In such case, the dependence of current on polarization is usually linear (not logarithmic): : This linear region is called ''polarization resistance'' due to its formal similarity toOhm's law
Ohm's law states that the electric current through a Electrical conductor, conductor between two Node (circuits), points is directly Proportionality (mathematics), proportional to the voltage across the two points. Introducing the constant of ...
.
Kinetics of corrosion
The pace at whichcorrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
develops is determined by the kinetics of the reactions involved, hence the electrical double layer
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
is critical.
Applying an overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly r ...
to an electrode causes the reaction to move in one direction, away from equilibrium. Tafel's law determines the new rate, and as long as the reaction kinetics are under control, the overpotential is proportional to the log of the corrosion current.
See also
*Overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly r ...
* Butler–Volmer equation
In electrochemistry, the Butler–Volmer equation (named after John Alfred Valentine Butler and Max Volmer), also known as Tibor Erdey-Grúz, Erdey-Grúz–Volmer equation, is one of the most fundamental relationships in electrochemical kinetics. I ...
* Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst ca ...
* Faradaic current
In electrochemistry, the faradaic current is the electric current generated by the reduction or oxidation of some chemical substance at an electrode. The net faradaic current is the algebraic sum of all the faradaic currents flowing through an i ...
* Faraday's laws of electrolysis
Faraday's laws of electrolysis are quantitative relationships based on the electrochemical research published by Michael Faraday in 1833.
First law
Michael Faraday reported that the mass () of a substance deposited or liberated at an electrod ...
References
Further reading
*External links
* {{Commons category-inline Chemical kinetics Electrochemical equations Physical chemistry