ternary compound on:
[Wikipedia]
[Google]
[Amazon]
In
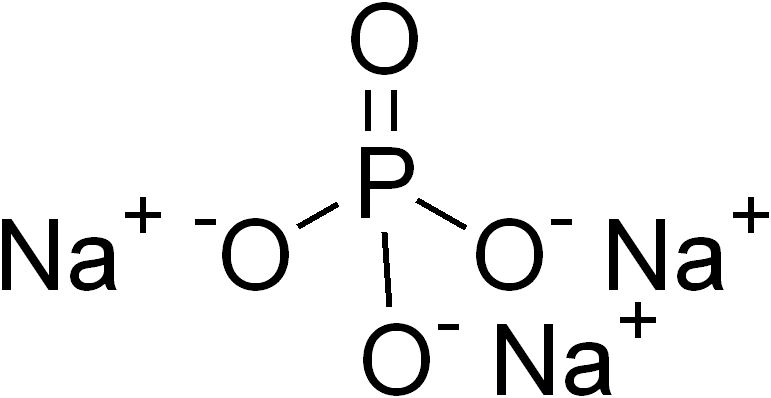 An example is sodium phosphate, . The
An example is sodium phosphate, . The
 Letting A and B represent cations and X an anion, these ternary groupings are organized by
Letting A and B represent cations and X an anion, these ternary groupings are organized by
C9 H10 O3 corresponds to more than 60 ternary compounds. F. K. Beilstein ''Handbuch der organischen Chemie'', page 58 Theodor Benfey (1964) ''From Vital Force to Structural Formulas'', page 12, Houghton Mifflin Company
inorganic chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subj ...
and materials chemistry, a ternary compound or ternary phase is a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
containing three different elements.
While some ternary compounds are molecular, ''e.g.'' chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
(), more typically ternary phases refer to extended solids. The perovskites are a famous example.
Binary phases, with only two elements, have lower degrees of complexity than ternary phases. With four elements, quaternary phases are more complex.
The number of isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s of a ternary compound provide a distinction between inorganic and organic chemistry: "In inorganic chemistry one or, at most, only a few compounds composed of any two or three elements were known, whereas in organic chemistry the situation was very different."
Ternary crystalline compounds
 An example is sodium phosphate, . The
An example is sodium phosphate, . The sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
ion has a charge of 1+ and the phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
ion has a charge of 3–. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of a ternary compound is calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
, . In naming and writing the formulae for ternary compounds, rules are similar to binary compounds.
Classifications of ternary crystals
According to Rustum Roy and Olaf Müller, Rustum Roy & Olaf Müller (1974) ''The Major Ternary Structural Families'', Springer-Verlag "the chemistry of the entire mineral world informs us that ''chemical'' complexity can easily be accommodated within structural simplicity." The example ofzircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of th ...
is cited, where various metal atoms are replaced in the same crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
. "The structural entity ... remains ternary in character and is able to accommodate an enormous range of chemical elements." The great variety of ternary compounds is therefore reduced to relatively few structures: "By dealing with approximately ten ternary structural groupings we can cover the most important structures of science and technology specific to the non-metallics world. It is a remarkable instance of nature's simplexity."
 Letting A and B represent cations and X an anion, these ternary groupings are organized by
Letting A and B represent cations and X an anion, these ternary groupings are organized by stoichiometric
Stoichiometry () is the relationships between the masses of reactants and products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total m ...
types , , and .
A ternary compound of type may be in the class of olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
, the spinel group, or phenakite. Examples include , β-, and .
One of type may be of the class of zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of th ...
, scheelite
Scheelite is a calcium tungstate mineral with the chemical formula Ca W O4. It is an important ore of tungsten (wolfram). Scheelite is originally named after Swedish chemist Carl Wilhelm Scheele (1742–1786). Well-formed crystals are sought ...
, barite or an ordered silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
derivative
In mathematics, the derivative is a fundamental tool that quantifies the sensitivity to change of a function's output with respect to its input. The derivative of a function of a single variable at a chosen input value, when it exists, is t ...
.
In the class of ternary compounds, there are the structures of perovskite (structure), calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
, pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron ( ...
s, corundum
Corundum is a crystalline form of aluminium oxide () typically containing traces of iron, titanium, vanadium, and chromium. It is a rock (geology), rock-forming mineral. It is a naturally transparency and translucency, transparent material, but ...
and hexagonal types.
Other ternary compounds are described as crystals of types , , , , and .
Ternary semiconductors
A particular class of ternary compounds are the ternary semiconductors, particularly within the III-V semiconductor family. In this type of semiconductor, the ternary can be considered to be an alloy of the two binary endpoints. Varying the composition between the endpoints allows both the lattice constant and the energybandgap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the ...
to be adjusted to produce the properties desired, for example, in emitting light (for example, as a LED) or absorbing light (as a photodetector
Photodetectors, also called photosensors, are devices that detect light or other forms of electromagnetic radiation and convert it into an electrical signal. They are essential in a wide range of applications, from digital imaging and optical ...
or a photovoltaic cell
A solar cell, also known as a photovoltaic cell (PV cell), is an electronic device that converts the energy of light directly into electricity by means of the photovoltaic effect.
). An example would be the semiconductor indium gallium arsenide
Indium gallium arsenide (InGaAs) (alternatively gallium indium arsenide, GaInAs) is a ternary alloy (chemical compound) of indium arsenide (InAs) and gallium arsenide (GaAs). Indium and gallium are Group 13 element, group III elements of the peri ...
(), a material with band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
dependent on In/Ga ratio.
Important examples of ternary semiconductors can also be found in other semiconductor families, such as the II-VI family (''e.g.'', Mercury cadmium telluride, ), or the I-II-VI2 family, with examples such as .
Organics
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s and carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s are ternary compounds with carbon, oxygen, and hydrogen. Other organic ternary compounds replace oxygen with another atom to form functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s.
The multiplicity of ternary compounds based on has been noted. For example, See also
* Binary compound * Mitscherlich's law of isomorphism * Quaternary phaseReferences
{{DEFAULTSORT:Ternary Compound Chemical compounds