Technetium Heptoxide on:
[Wikipedia]
[Google]
[Amazon]
Technetium is a
 The most prevalent form of technetium that is easily accessible is
The most prevalent form of technetium that is easily accessible is
 The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The
The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The
^_U -> ce^_I + ^_Y + 2^_n
^_Y -> beta^-1.47\,\ce] ^_Zr -> beta^-2.1\,\ce] ^_Nb -> beta^-15.0\,\ce] ^_Mo -> beta^-65.94\,\ce] ^_Tc -> beta^-211,100\,\ce] ^_Ru
Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m is decayed by the time that the fission products are separated from the major  Almost two-thirds of the world's supply comes from two reactors; the
Almost two-thirds of the world's supply comes from two reactors; the
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Tc and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
43. It is the lightest element whose isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
are all radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
. Technetium and promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
are the only radioactive elements whose neighbours in the sense of atomic number are both stable. All available technetium is produced as a synthetic element
A synthetic element is a known chemical element that does not occur naturally on Earth: it has been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, it i ...
. Naturally occurring technetium is a spontaneous fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
in uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
and thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
ore (the most common source), or the product of neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
in molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
ores. This silvery gray, crystalline transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
lies between manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
and rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
in group 7 of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
Many of technetium's properties had been predicted by Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
before it was discovered; Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium became the first predominantly artificial element to be produced, hence its name (from the Greek ', 'artificial', +
One short-lived gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
–emitting nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
, technetium-99m
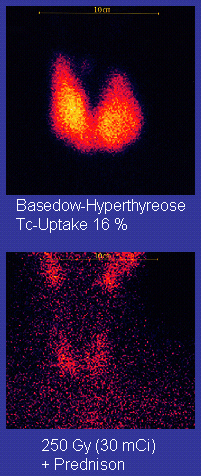
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
, is used in nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
for a wide variety of tests, such as bone cancer diagnoses. The ground state of the nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
technetium-99
Technetium-99 (99Tc) is an isotope of technetium that decays with a half-life of 211,000 years to stable ruthenium-99, emitting beta particles, but no gamma rays. It is the most significant long-lived fission product of uranium fission, produci ...
is used as a gamma ray–free source of beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
s. Long-lived technetium isotopes produced commercially are byproducts of the fission of uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
in nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction. They are used for commercial electricity, marine propulsion, weapons production and research. Fissile nuclei (primarily uranium-235 or plutonium-2 ...
and are extracted from nuclear fuel rods. Because even the longest-lived isotope of technetium has a relatively short half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
(4.21 million years), the 1952 detection of technetium in red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The stellar atmosphere, outer atmosphere is inflated and tenuous, making the radius large and the surface t ...
s helped to prove that stars can produce heavier elements.
History
Early assumptions
From the 1860s through 1871, early forms of the periodic table proposed byDmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
contained a gap between molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
(element 42) and ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
(element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
and have similar chemical properties. Mendeleev gave it the provisional name ''eka-manganese'' (from ''eka'', the Sanskrit
Sanskrit (; stem form ; nominal singular , ,) is a classical language belonging to the Indo-Aryan languages, Indo-Aryan branch of the Indo-European languages. It arose in northwest South Asia after its predecessor languages had Trans-cultural ...
word for ''one'') because it was one place down from the known element manganese.
Early misidentifications
Many early researchers, both before and after the periodic table was published, were eager to be the first to discover and name the missing element. Its location in the table suggested that it should be easier to find than other undiscovered elements. This turned out not to be the case, due to technetium's radioactivity.Irreproducible results
German chemistsWalter Noddack
Walter Noddack (17 August 1893 – 7 December 1960) was a German chemist. He, Ida Tacke (who later married Noddack), and Otto Berg reported the discovery of element 43 and element 75 in 1925.
Rhenium
They named element 75 rhenium (Latin ''Rh ...
, Otto Berg, and Ida Tacke
Ida Noddack (25 February 1896 – 24 September 1978), ''née'' Tacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg (scientist), Otto ...
reported the discovery of element 75 and element 43 in 1925, and named element 43 ''masurium'' (after Masuria
Masuria ( ; ; ) is an ethnographic and geographic region in northern and northeastern Poland, known for its 2,000 lakes. Masuria occupies much of the Masurian Lake District. Administratively, it is part of the Warmian–Masurian Voivodeship (ad ...
in eastern Prussia
Prussia (; ; Old Prussian: ''Prūsija'') was a Germans, German state centred on the North European Plain that originated from the 1525 secularization of the Prussia (region), Prussian part of the State of the Teutonic Order. For centuries, ...
, now in Poland
Poland, officially the Republic of Poland, is a country in Central Europe. It extends from the Baltic Sea in the north to the Sudetes and Carpathian Mountains in the south, bordered by Lithuania and Russia to the northeast, Belarus and Ukrai ...
, the region where Walter Noddack's family originated). This name caused significant resentment in the scientific community, because it was interpreted as referring to a series
Series may refer to:
People with the name
* Caroline Series (born 1951), English mathematician, daughter of George Series
* George Series (1920–1995), English physicist
Arts, entertainment, and media
Music
* Series, the ordered sets used i ...
of victories of the German army over the Russian army in the Masuria region during World War I; as the Noddacks remained in their academic positions while the Nazis were in power, suspicions and hostility against their claim for discovering element 43 continued. The group bombarded columbite
Columbite, also called niobite, niobite-tantalite and columbate, with a general chemical formula of , is a black mineral group that is an ore of niobium. It has a submetallic luster, a high density, and is a niobate of iron and manganese. Niobite ...
with a beam of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s and deduced element 43 was present by examining X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
emission spectrogram
A spectrogram is a visual representation of the spectrum of frequencies of a signal as it varies with time.
When applied to an audio signal, spectrograms are sometimes called sonographs, voiceprints, or voicegrams. When the data are represen ...
s. The wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
of the X-rays produced is related to the atomic number by a formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
derived by Henry Moseley
Henry Gwyn Jeffreys Moseley (; 23 November 1887 – 10 August 1915) was an English physicist, whose contribution to the science of physics was the justification from physical laws of the previous empirical and chemical concept of the atomic numb ...
in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error. Still, in 1933, a series of articles on the discovery of elements quoted the name ''masurium'' for element 43. Some more recent attempts have been made to rehabilitate the Noddacks' claims, but they are disproved by Paul Kuroda's study on the amount of technetium that could have been present in the ores they studied: it could not have exceeded of ore, and thus would have been undetectable by the Noddacks' methods.
Official discovery and later history
Thediscovery
Discovery may refer to:
* Discovery (observation), observing or finding something unknown
* Discovery (fiction), a character's learning something unknown
* Discovery (law), a process in courts of law relating to evidence
Discovery, The Discovery ...
of element 43 was finally confirmed in a 1937 experiment at the University of Palermo
The University of Palermo () is a public university, public research university in Palermo, Italy. It was founded in 1806, and is currently organized in 12 Faculties.
History
The University of Palermo was officially founded in 1806, although it ...
in Sicily by Carlo Perrier and Emilio Segrè
Emilio Gino Segrè ( ; ; 1 February 1905 – 22 April 1989) was an Italian-American nuclear physicist and radiochemist who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was award ...
. In mid-1936, Segrè visited the United States, first Columbia University
Columbia University in the City of New York, commonly referred to as Columbia University, is a Private university, private Ivy League research university in New York City. Established in 1754 as King's College on the grounds of Trinity Churc ...
in New York and then the Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a Federally funded research and development centers, federally funded research and development center in the Berkeley Hills, hills of Berkeley, California, United States. Established i ...
in California. He persuaded cyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
inventor Ernest Lawrence
Ernest Orlando Lawrence (August 8, 1901 – August 27, 1958) was an American accelerator physicist who received the Nobel Prize in Physics in 1939 for his invention of the cyclotron. He is known for his work on uranium-isotope separation for ...
to let him take back some discarded cyclotron parts that had become radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
. Lawrence mailed him a molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
foil that had been part of the deflector in the cyclotron.
Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed from an element with the atomic number 43, which they did. University of Palermo
The University of Palermo () is a public university, public research university in Palermo, Italy. It was founded in 1806, and is currently organized in 12 Faculties.
History
The University of Palermo was officially founded in 1806, although it ...
officials wanted them to name their discovery , after the Latin name for Palermo
Palermo ( ; ; , locally also or ) is a city in southern Italy, the capital (political), capital of both the autonomous area, autonomous region of Sicily and the Metropolitan City of Palermo, the city's surrounding metropolitan province. The ...
, '. In 1947, element 43 was named after the Greek
Greek may refer to:
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group
*Greek language, a branch of the Indo-European language family
**Proto-Greek language, the assumed last common ancestor of all kno ...
word (), meaning 'artificial', since it was the first element to be artificially produced.
Segrè returned to Berkeley and met Glenn T. Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
. They isolated the metastable isotope technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
, which is now used in some ten million medical diagnostic procedures annually.
In 1952, the astronomer Paul W. Merrill detected the spectral signature
Spectral signature is the variation of reflectance or emittance of a material with respect to wavelengths (i.e., reflectance/emittance as a function of wavelength). The spectral signature of stars indicates the composition of the stellar atmosph ...
of technetium (specifically wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
s of 403.1 nm, 423.8 nm, 426.2 nm, and 429.7 nm) in light from S-type red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The stellar atmosphere, outer atmosphere is inflated and tenuous, making the radius large and the surface t ...
s. The stars were near the end of their lives but were rich in the short-lived element, which indicated that it was being produced in the stars by nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s. That evidence bolstered the hypothesis that heavier elements are the product of nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
in stars. More recently, such observations provided evidence that elements are formed by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
in the s-process
The slow neutron-capture process, or ''s''-process, is a series of nuclear reactions, reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynt ...
.
Since that discovery, there have been many searches in terrestrial materials for natural sources of technetium. In 1962, technetium-99 was isolated and identified in pitchblende
Uraninite, also known as pitchblende, is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2 but because of oxidation typically contains variable proportions of U3O8. Radioactive decay of the urani ...
from the Belgian Congo
The Belgian Congo (, ; ) was a Belgian colonial empire, Belgian colony in Central Africa from 1908 until independence in 1960 and became the Republic of the Congo (Léopoldville). The former colony adopted its present name, the Democratic Repu ...
in very small quantities (about 0.2 ng/kg), where it originates as a spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
product of uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
. The natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The idea of a nuclear reactor existing ''in situ'' within an ore body moderated by groundwater was briefly explored by Paul Kuroda in 19 ...
in Oklo
Oklo is a region near Franceville in the Haut-Ogooué Province of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a French colony when prospectors from the Comm ...
contains evidence that significant amounts of technetium-99 were produced and have since decayed into ruthenium-99.
Characteristics
Physical properties
Technetium is a silvery-gray radioactivemetal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
with an appearance similar to platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, commonly obtained as a gray powder. The crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of the bulk pure metal is hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
close-packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occ ...
. Atomic technetium has characteristic emission lines
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used ...
at wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
s of 363.3 nm, 403.1 nm, 426.2 nm, 429.7 nm, and 485.3 nm. The unit cell parameters of the orthorhombic Tc metal were reported when Tc is contaminated with carbon ( = 0.2805(4), = 0.4958(8), = 0.4474(5)·nm for Tc-C with 1.38 wt% C and = 0.2815(4), = 0.4963(8), = 0.4482(5)·nm for Tc-C with 1.96 wt% C ). The metal form is slightly paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
, meaning its magnetic dipoles align with external magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
s, but will assume random orientations once the field is removed. Pure, metallic, single-crystal technetium becomes a type-II superconductor
In superconductivity, a type-II superconductor is a superconductor that exhibits an intermediate phase of mixed ordinary and superconducting properties at intermediate temperature and fields above the superconducting phases.
It also features the ...
at temperatures below .
Below this temperature, technetium has a very high magnetic penetration depth, greater than any other element except niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
.
Chemical properties
Technetium is located in group 7 of the periodic table, betweenrhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
. As predicted by the periodic law
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain elements when grouped by period and/or group. They were discovered by the Russian chemist Dmitri Mendeleev in 1863. ...
, its chemical properties are between those two elements. Of the two, technetium more closely resembles rhenium, particularly in its chemical inertness and tendency to form covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s. This is consistent with the tendency of period 5 elements to resemble their counterparts in period 6 more than period 4 due to the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii and ionic radii of the elements in the lanthanide series, from left to right. It is caused by the poor shielding effect of nuclear charge by the 4f electrons alo ...
. Unlike manganese, technetium does not readily form cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s (ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s with net positive charge). Technetium exhibits nine oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s from −1 to +7, with +4, +5, and +7 being the most common. Technetium dissolves in aqua regia
Aqua regia (; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar concentration, molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but i ...
, nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, and concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, but ''not'' in hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
of any concentration.
Metallic technetium slowly tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
es in moist air and, in powder form, burns in oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. When reacting with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
at high pressure, it forms the non-stoichiometric
Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having chemical element, elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); ...
hydride TcH and while reacting with carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
it forms TcC, with cell parameter 0.398 nm.
Technetium can catalyse the destruction of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
by nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, and this property is due to its multiplicity of valencies. This caused a problem in the separation of plutonium from uranium in nuclear fuel processing, where hydrazine is used as a protective reductant to keep plutonium in the trivalent rather than the more stable tetravalent state. The problem was exacerbated by the mutually enhanced solvent extraction of technetium and zirconium at the previous stage, and required a process modification.
Compounds
Pertechnetate and other derivatives
sodium pertechnetate
Sodium pertechnetate is the inorganic compound with the formula NaTcO4. This colourless salt contains the pertechnetate anion, that has slightly distorted tetrahedron symmetry both at 296 K and at 100 K while the coordination polyhedron of ths ...
, Na cO4 The majority of this material is produced by radioactive decay from sup>99MoO4sup>2−:
Pertechnetate
The pertechnetate ion () is an oxyanion with the chemical formula . It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the Technetium-99m, 99mTc isotope ( ...
() is only weakly hydrated in aqueous solutions, and it behaves analogously to perchlorate anion, both of which are tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
. Unlike permanganate
A permanganate () is a chemical compound with the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition ...
(), it is only a weak oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
.
Related to pertechnetate is technetium heptoxide. This pale-yellow, volatile solid is produced by oxidation of Tc metal and related precursors:
It is a molecular metal oxide, analogous to manganese heptoxide
Manganese(VII) oxide (manganese heptoxide) is an inorganic compound with the formula . Manganese heptoxide is a volatile liquid with an oily consistency. It is a highly reactive and powerful oxidizer that reacts explosively with nearly any organi ...
. It adopts a centrosymmetric
In crystallography, a centrosymmetric point group contains an inversion center as one of its symmetry elements. In such a point group, for every point (x, y, z) in the unit cell there is an indistinguishable point (-x, -y, -z). Such point grou ...
structure with two types of Tc−O bonds with 167 and 184 pm bond lengths.
Technetium heptoxide hydrolyzes to pertechnetate and pertechnetic acid
Pertechnetic acid (HTcO4) is a compound of technetium that is produced by reacting technetium(VII) oxide (Tc2O7) with water or reacting Tc metal or TcO2 with strong oxidizing acids, such as nitric acid, mixture of concentrated sulfuric acid wit ...
, depending on the pH:
HTcO4 is a strong acid. In concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, cO4sup>− converts to the octahedral form TcO3(OH)(H2O)2, the conjugate base of the hypothetical triaquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
cO3(H2O)3sup>+.
Other chalcogenide derivatives
Technetium forms a dioxide,disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In inorg ...
, diselenide
A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.
Inorganic selenides
Th ...
, and di telluride. An ill-defined Tc2S7 forms upon treating pertechnate with hydrogen sulfide. It thermally decomposes into disulfide and elemental sulfur. Similarly the dioxide can be produced by reduction of the Tc2O7.
Unlike the case for rhenium, a trioxide has not been isolated for technetium. However, TcO3 has been identified in the gas phase using mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
.
Simple hydride and halide complexes
Technetium forms the complex . The potassium salt isisostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
with . At high pressure formation of TcH1.3 from elements was also reported.
 The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The
The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s range from Tc(VI) to Tc(II). Technetium halides exhibit different structure types, such as molecular octahedral complexes, extended chains, layered sheets, and metal clusters arranged in a three-dimensional network. These compounds are produced by combining the metal and halogen or by less direct reactions.
TcCl4 is obtained by chlorination of Tc metal or Tc2O7. Upon heating, TcCl4 gives the corresponding Tc(III) and Tc(II) chlorides.
The structure of TcCl4 is composed of infinite zigzag chains of edge-sharing TcCl6 octahedra. It is isomorphous to transition metal tetrachlorides of zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, and platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
.
Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure. It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides cX6sup>2− (X = F, Cl, Br, I), which adopt octahedral molecular geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The o ...
. More reduced halides form anionic clusters with Tc–Tc bonds. The situation is similar for the related elements of Mo, W, Re. These clusters have the nuclearity Tc4, Tc6, Tc8, and Tc13. The more stable Tc6 and Tc8 clusters have prism shapes where vertical pairs of Tc atoms are connected by triple bonds and the planar atoms by single bonds. Every technetium atom makes six bonds, and the remaining valence electrons can be saturated by one axial and two bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
halogen atoms such as chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
or bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
.
Coordination and organometallic complexes
Technetium forms a variety ofcoordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es with organic ligands. Many have been well-investigated because of their relevance to nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
.
Technetium forms a variety of compounds with Tc–C bonds, i.e. organotechnetium complexes. Prominent members of this class are complexes with CO, arene, and cyclopentadienyl ligands. The binary carbonyl Tc2(CO)10 is a white volatile solid. In this molecule, two technetium atoms are bound to each other; each atom is surrounded by octahedra
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
of five carbonyl ligands. The bond length between technetium atoms, 303 pm, is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
s are formed by technetium's congeners, manganese and rhenium. Interest in organotechnetium compounds has also been motivated by applications in nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
. Technetium also forms aquo-carbonyl complexes, one prominent complex being c(CO)3(H2O)3sup>+, which are unusual compared to other metal carbonyls.
Isotopes
Technetium, withatomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
''Z'' = 43, is the lowest-numbered element in the periodic table for which all isotopes are radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
. The second-lightest exclusively radioactive element, promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
, has atomic number 61. Atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. Aft ...
with an odd number of proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s are less stable than those with even numbers, even when the total number of nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number.
Until the 1960s, nucleons were thought to be ele ...
s (protons + neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s) is even, and odd numbered elements have fewer stable isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s.
The most stable radioactive isotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
are technetium-97 with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of million years and technetium-98 with million years; current measurements of their half-lives give overlapping confidence intervals corresponding to one standard deviation
In statistics, the standard deviation is a measure of the amount of variation of the values of a variable about its Expected value, mean. A low standard Deviation (statistics), deviation indicates that the values tend to be close to the mean ( ...
and therefore do not allow a definite assignment of technetium's most stable isotope. The next most stable isotope is technetium-99, which has a half-life of 211,100 years. Thirty-four other radioisotopes have been characterized with mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
s ranging from 86 to 122. Most of these have half-lives that are less than an hour, the exceptions being technetium-93 (2.73 hours), technetium-94 (4.88 hours), technetium-95 (20 hours), and technetium-96 (4.3 days).
The primary decay mode
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
for isotopes lighter than technetium-98 (98Tc) is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Th ...
, producing molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
(''Z'' = 42). For technetium-98 and heavier isotopes, the primary mode is beta emission
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron t ...
(the emission of an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
or positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
), producing ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
(''Z'' = 44), with the exception that technetium-100 can decay both by beta emission and electron capture.
Technetium also has numerous nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
s, which are isotopes with one or more excited nucleons. Technetium-97m (97mTc; "m" stands for metastability
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
) is the most stable, with a half-life of 91 days and excitation energy 0.0965 MeV.
This is followed by technetium-95m (61 days, 0.03 MeV), and technetium-99m (6.01 hours, 0.142 MeV).
Technetium-99 (99Tc) is a major product of the fission of uranium-235 (235U), making it the most common and most readily available isotope of technetium. One gram of technetium-99 produces per second (in other words, the specific activity
Specific activity (symbol ''a'') is the activity per unit mass of a radionuclide and is a physical property of that radionuclide.
It is usually given in units of becquerel per kilogram (Bq/kg), but another commonly used unit of specific activi ...
of 99Tc is 0.62 G Bq/g).
Occurrence and production
Technetium occurs naturally in the Earth's crust in minute concentrations of about 0.003 parts per trillion. Technetium is so rare because thehalf-lives Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* '' Half Life: A Parable for t ...
of 97Tc and 98Tc are only More than a thousand of such periods have passed since the formation of the Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
, so the probability of survival of even one atom of primordial technetium is effectively zero. However, small amounts exist as spontaneous fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
s in uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
s. A kilogram of uranium contains an estimated 1 nanogram
To help compare different ''Order of magnitude, orders of magnitude'', the following lists describe various ''mass'' levels between 10−67 kilogram, kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thi ...
, equivalent to ten trillion atoms, of technetium.
Some red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The stellar atmosphere, outer atmosphere is inflated and tenuous, making the radius large and the surface t ...
stars with the spectral types S, M, and N display a spectral absorption line indicating the presence of technetium. These red giants are known informally as technetium star
A technetium star, or more properly a Tc-rich star, is a star whose stellar spectrum contains absorption lines of the radioactive metal technetium. The most stable isotope of technetium is Tc with a half-life of 4.21 million years: too short a tim ...
s.
Fission waste product
In contrast to the rare natural occurrence, bulk quantities of technetium-99 are produced each year from spent nuclear fuel rods, which contain various fission products. The fission of a gram ofuranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s yields 27 mg of technetium-99, giving technetium a fission product yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
# Individual i ...
of 6.1%. Other fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissil ...
isotopes produce similar yields of technetium, such as 4.9% from uranium-233
Uranium-233 ( or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully ...
and 6.21% from plutonium-239
Plutonium-239 ( or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main iso ...
. An estimated 49,000 T Bq (78 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the sh ...
) of technetium was produced in nuclear reactors between 1983 and 1994, by far the dominant source of terrestrial technetium.
Only a fraction of the production is used commercially.
Technetium-99 is produced by the nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
of both uranium-235 and plutonium-239. It is therefore present in radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
and in the nuclear fallout
Nuclear fallout is residual radioactive material that is created by the reactions producing a nuclear explosion. It is initially present in the mushroom cloud, radioactive cloud created by the explosion, and "falls out" of the cloud as it is ...
of fission bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear expl ...
explosions. Its decay, measured in becquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as an activity of one per second, on average, for aperiodic activity events referred to a radionuclide. For applicatio ...
s per amount of spent fuel, is the dominant contributor to nuclear waste radioactivity after about after the creation of the nuclear waste. From 1945–1994, an estimated 160 T Bq (about 250 kg) of technetium-99 was released into the environment during atmospheric nuclear test
Nuclear weapons tests are experiments carried out to determine the performance of nuclear weapons and the effects of their explosion. Nuclear testing is a sensitive political issue. Governments have often performed tests to signal strength. Bec ...
s.
The amount of technetium-99 from nuclear reactors released into the environment up to 1986 is on the order of 1000 TBq (about 1600 kg), primarily by nuclear fuel reprocessing
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
* Nuclear engineering
* Nuclear physics
* Nuclear power
* Nuclear reactor
* Nuclear weapon
* Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
* Nuclear space
* ...
; most of this was discharged into the sea. Reprocessing methods have reduced emissions since then, but as of 2005 the primary release of technetium-99 into the environment is by the Sellafield
Sellafield, formerly known as Windscale, is a large multi-function nuclear site close to Seascale on the coast of Cumbria, England. As of August 2022, primary activities are nuclear waste storage, nuclear waste processing and storage and nucle ...
plant, which released an estimated 550 TBq (about 900 kg) from 1995 to 1999 into the Irish Sea
The Irish Sea is a body of water that separates the islands of Ireland and Great Britain. It is linked to the Celtic Sea in the south by St George's Channel and to the Inner Seas off the West Coast of Scotland in the north by the North Ch ...
.
From 2000 onwards the amount has been limited by regulation to 90 TBq (about 140 kg) per year.
Discharge of technetium into the sea resulted in contamination of some seafood with minuscule quantities of this element. For example, European lobster
''Homarus gammarus'', known as the European lobster or common lobster, is a species of lobster, clawed lobster from the eastern Atlantic Ocean, Mediterranean Sea and parts of the Black Sea. It is closely related to the American lobster, ''H.&nbs ...
and fish from west Cumbria
Cumbria ( ) is a ceremonial county in North West England. It borders the Scottish council areas of Dumfries and Galloway and Scottish Borders to the north, Northumberland and County Durham to the east, North Yorkshire to the south-east, Lancash ...
contain about 1 Bq/kg of technetium.
Fission product for commercial use
Themetastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
isotope technetium-99m is continuously produced as a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
from the fission of uranium or plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s:
actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s in conventional nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the ...
. The liquid left after plutonium–uranium extraction (PUREX
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. It is based on liquid–liquid extraction ion-exchange. PUREX is the '' de facto'' standard aqueous nuclear reproc ...
) contains a high concentration of technetium as but almost all of this is technetium-99, not technetium-99m.
The vast majority of the technetium-99m used in medical work is produced by irradiating dedicated highly enriched uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238 ...
targets in a reactor, extracting molybdenum-99 from the targets in reprocessing facilities, and recovering at the diagnostic center the technetium-99m produced upon decay of molybdenum-99. Molybdenum-99 in the form of molybdate is adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
onto acid alumina () in a shielded column chromatograph inside a technetium-99m generator
A technetium-99m generator, or colloquially a technetium cow or moly cow, is a device used to extract the metastable isotope 99mTc of technetium from a decaying sample of molybdenum-99. 99Mo has a half-life of 66 hours and can be easily tran ...
("technetium cow", also occasionally called a "molybdenum cow"). Molybdenum-99 has a half-life of 67 hours, so short-lived technetium-99m (half-life: 6 hours), which results from its decay, is being constantly produced. The soluble pertechnetate
The pertechnetate ion () is an oxyanion with the chemical formula . It is often used as a convenient water-soluble source of isotopes of the radioactive element technetium (Tc). In particular it is used to carry the Technetium-99m, 99mTc isotope ( ...
can then be chemically extracted by elution
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent: washing of loaded ion-exchange resins to remove captured ions, or eluting proteins or other biopolymers from an el ...
using a saline solution
Saline (also known as saline solution) is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, i ...
. A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.
 Almost two-thirds of the world's supply comes from two reactors; the
Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor
The National Research Universal (NRU) reactor was a 135 MW nuclear research reactor built in the Chalk River Laboratories, Ontario, one of Canada’s national science facilities. It was a multipurpose science facility that served three main roles. ...
at Chalk River Laboratories
Chalk River Laboratories (; also known as CRL, Chalk River Labs and formerly Chalk River Nuclear Laboratories, CRNL) is a Canadian nuclear research facility in Deep River, about north-west of Ottawa.
CRL is a site of significant research and ...
in Ontario, Canada, and the High Flux Reactor at Nuclear Research and Consultancy Group in Petten, Netherlands. All major reactors that produce technetium-99m were built in the 1960s and are close to the end of life. The two new Canadian Multipurpose Applied Physics Lattice Experiment reactors planned and built to produce 200% of the demand of technetium-99m relieved all other producers from building their own reactors. With the cancellation of the already tested reactors in 2008, the future supply of technetium-99m became problematic.
Waste disposal
The long half-life of technetium-99 and its potential to formanionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
species creates a major concern for long-term disposal of radioactive waste. Many of the processes designed to remove fission products in reprocessing plants aim at cationic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
species such as caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
(e.g., caesium-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nucle ...
) and strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
(e.g., strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine a ...
). Hence the pertechnetate escapes through those processes. Current disposal options favor burial
Burial, also known as interment or inhumation, is a method of final disposition whereby a dead body is placed into the ground, sometimes with objects. This is usually accomplished by excavating a pit or trench, placing the deceased and objec ...
in continental, geologically stable rock. The primary danger with such practice is the likelihood that the waste will contact water, which could leach radioactive contamination into the environment. The anionic pertechnetate and iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
tend not to adsorb into the surfaces of minerals, and are likely to be washed away. By comparison plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, and caesium
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only f ...
tend to bind to soil particles. Technetium could be immobilized by some environments, such as microbial activity in lake bottom sediments, and the environmental chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as ...
of technetium is an area of active research.
An alternative disposal method, transmutation, has been demonstrated at CERN
The European Organization for Nuclear Research, known as CERN (; ; ), is an intergovernmental organization that operates the largest particle physics laboratory in the world. Established in 1954, it is based in Meyrin, western suburb of Gene ...
for technetium-99. In this process, the technetium (technetium-99 as a metal target) is bombarded with neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s to form the short-lived technetium-100 (half-life = 16 seconds) which decays by beta decay to stable ruthenium
Ruthenium is a chemical element; it has symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is unreactive to most chem ...
-100. If recovery of usable ruthenium is a goal, an extremely pure technetium target is needed; if small traces of the minor actinide
Minor may refer to:
Common meanings
* Minor (law), a person not under the age of certain legal activities.
* Academic minor, a secondary field of study in undergraduate education
Mathematics
* Minor (graph theory), a relation of one graph to ...
s such as americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
and curium
Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inten ...
are present in the target, they are likely to undergo fission and form more fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
s which increase the radioactivity of the irradiated target. The formation of ruthenium-106 (half-life 374 days) from the 'fresh fission' is likely to increase the activity of the final ruthenium metal, which will then require a longer cooling time after irradiation before the ruthenium can be used.
The actual separation of technetium-99 from spent nuclear fuel is a long process. During fuel reprocessing, it comes out as a component of the highly radioactive waste liquid. After sitting for several years, the radioactivity reduces to a level where extraction of the long-lived isotopes, including technetium-99, becomes feasible. A series of chemical processes yields technetium-99 metal of high purity.
Neutron activation
Molybdenum-99
Molybdenum (42Mo) has 39 known isotopes, ranging in atomic mass from 81 to 119, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum ...
, which decays to form technetium-99m, can be formed by the neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emi ...
of molybdenum-98. When needed, other technetium isotopes are not produced in significant quantities by fission, but are manufactured by neutron irradiation of parent isotopes (for example, technetium-97 can be made by neutron irradiation of ruthenium-96).
Particle accelerators
The feasibility of technetium-99m production with the 22-MeV-proton bombardment of a molybdenum-100 target in medical cyclotrons following the reaction 100Mo(p,2n)99mTc was demonstrated in 1971. The recent shortages of medical technetium-99m reignited the interest in its production by proton bombardment of isotopically enriched (>99.5%) molybdenum-100 targets. Other techniques are being investigated for obtaining molybdenum-99 from molybdenum-100 via (n,2n) or (γ,n) reactions in particle accelerators.Applications
Nuclear medicine and biology

Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used Radiophar ...
("m" indicates that this is a metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
nuclear isomer) is used in radioactive isotope medical tests
A medical test is a medical procedure performed to screening (medicine), detect, medical diagnosis, diagnose, or monitoring (medicine), monitor diseases, disease processes, susceptibility, or to determine a course of treatment. Medical tests suc ...
. For example, technetium-99m is a radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
that medical imaging equipment tracks in the human body. It is well suited to the role because it emits readily detectable 140 keV
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one volt in vacuum. When us ...
gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). The chemistry of technetium allows it to be bound to a variety of biochemical compounds, each of which determines how it is metabolized and deposited in the body, and this single isotope can be used for a multitude of diagnostic tests. More than 50 common radiopharmaceuticals
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which ...
are based on technetium-99m for imaging and functional studies of the brain
The brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head (cephalization), usually near organs for ...
, heart muscle, thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by ...
, lungs
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syste ...
, liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
, gall bladder
In vertebrates, the gallbladder, also known as the cholecyst, is a small hollow organ where bile is stored and concentrated before it is released into the small intestine. In humans, the pear-shaped gallbladder lies beneath the liver, althoug ...
, kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and rig ...
s, skeleton
A skeleton is the structural frame that supports the body of most animals. There are several types of skeletons, including the exoskeleton, which is a rigid outer shell that holds up an organism's shape; the endoskeleton, a rigid internal fra ...
, blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
, and tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s.
The longer-lived isotope, technetium-95m with a half-life of 61 days, is used as a radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
to study the movement of technetium in the environment and in plant and animal systems.
Industrial and chemical
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma rays. Moreover, its long half-life means that this emission decreases very slowly with time. It can also be extracted to a high chemical and isotopic purity from radioactive waste. For these reasons, it is a U.S.National Institute of Standards and Technology
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into Outline of p ...
(NIST) standard beta emitter, and is used for equipment calibration. Technetium-99 has also been proposed for optoelectronic devices and nanoscale
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing propertie ...
nuclear batteries.
Like rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, technetium can serve as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. In processes such as the dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
of isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor.
Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, an ...
, it is a far more effective catalyst than either rhenium or palladium. However, its radioactivity is a major problem in safe catalytic applications.
When steel is immersed in water, adding a small concentration (55 ppm) of potassium pertechnetate(VII) to the water protects the steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
from corrosion, even if the temperature is raised to . For this reason, pertechnetate has been used as an anodic corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
inhibitor for steel, although technetium's radioactivity poses problems that limit this application to self-contained systems. While (for example) can also inhibit corrosion, it requires a concentration ten times as high. In one experiment, a specimen of carbon steel was kept in an aqueous solution of pertechnetate for 20 years and was still uncorroded. The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer ( passivation). One theory holds that the pertechnetate reacts with the steel surface to form a layer of technetium dioxide which prevents further corrosion; the same effect explains how iron powder can be used to remove pertechnetate from water. The effect disappears rapidly if the concentration of pertechnetate falls below the minimum concentration or if too high a concentration of other ions is added.
As noted, the radioactive nature of technetium (3 MBq/L at the concentrations required) makes this corrosion protection impractical in almost all situations. Nevertheless, corrosion protection by pertechnetate ions was proposed (but never adopted) for use in boiling water reactor
A boiling water reactor (BWR) is a type of nuclear reactor used for the generation of electrical power. It is the second most common type of electricity-generating nuclear reactor after the pressurized water reactor (PWR).
BWR are thermal neutro ...
s.
Precautions and biological effect
Technetium plays no natural biological role and is not normally found in the human body. Technetium is produced in quantity by nuclear fission, and spreads more readily than many radionuclides. It appears to have low chemical toxicity. For example, no significant change in blood formula, body and organ weights, and food consumption could be detected for rats which ingested up to 15 μg of technetium-99 per gram of food for several weeks. In the body, technetium quickly gets converted to the stable ion, which is highly water-soluble and quickly excreted. The radiological toxicity of technetium (per unit of mass) is a function of compound, type of radiation for the isotope in question, and the isotope's half-life. All isotopes of technetium must be handled carefully. The most common isotope, technetium-99, is a weak beta emitter; such radiation is stopped by the walls of laboratory glassware. The primary hazard when working with technetium is inhalation of dust; suchradioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of Radioactive decay, radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is uni ...
in the lungs can pose a significant cancer risk. For most work, careful handling in a fume hood
A fume hood (sometimes called a fume cupboard or fume closet, not to be confused with Extractor hood) is a type of local exhaust ventilation (architecture), ventilation device that is designed to prevent users from being exposed to hazardous f ...
is sufficient, and a glove box
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hand ...
is not needed.
Being close to noble metals, technetium is not very susceptible to corrosion, and during biofouling, its ability to self-cleanse has been recorded due to its radiotoxic effect on biota.
Notes
References
S. Garg and B. Maheshwari, et al., Atomic Data and Nuclear Data Tables 150, 101546 (2023) https://doi.org/10.1016/j.adt.2022.101546Bibliography
* * * * *Further reading
* * * * * *External links
* {{Authority control Chemical elements Transition metals Synthetic elements Chemical elements predicted by Dmitri Mendeleev Chemical elements with hexagonal close-packed structure